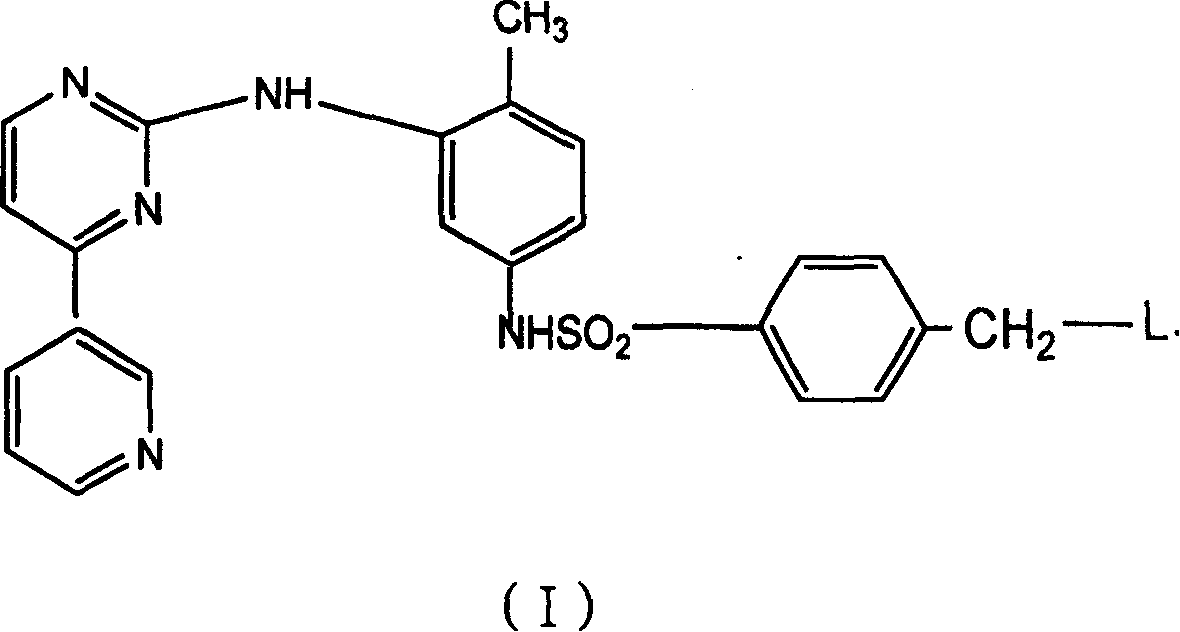
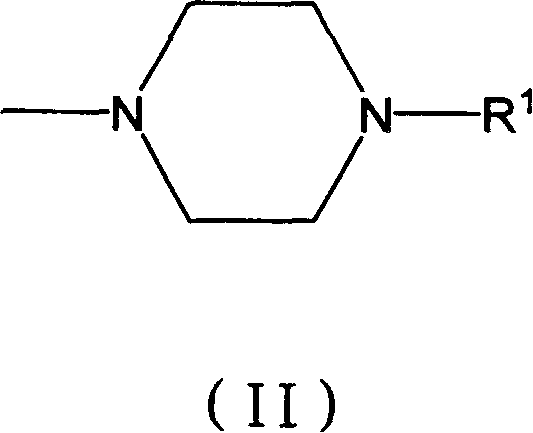
Novel pyrimidyl sulfamide compounds with anti-tumor activity and their salts
A technology for pyrimidine sulfonamides and compounds, applied in the field of new pyrimidine sulfonamide compounds and their salts, to achieve the effects of various administration routes, good crystallization, and increased water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1. Preparation of raw material compound 4-bromomethylbenzenesulfonyl chloride (VII):
[0043] 19.1g (0.1mol) of p-toluenesulfonyl chloride was dissolved in carbon tetrachloride (CCl 4 ), heating, when the reaction solution began to reflux, add bromosuccinimide (NBS, Shanghai Chemical Reagent Company) 18.69g (0.105mol) and catalytic amount free radical initiator 2,2'-bis Azaisobutyronitrile (AIBN, Shanghai Chemical Reagent Company) 2g. After the feeding was completed, the reaction reflux intensified, the liquid was red, and red gas was generated. After no gas was generated, the reaction was refluxed for another 1 hour, and the heating was stopped. Cool to room temperature and filter. After most of the solvent was evaporated from the filtrate under reduced pressure, 100ml of petroleum ether was added and stirred to precipitate a light yellow solid, which was filtered and dried in vacuo to obtain 24g of compound VII, melting point: 53-55°C.
Embodiment 2
[0044] Embodiment 2. Preparation of intermediate compound 4-bromomethyl-N-{4-methyl-3-[4-(3-pyridyl)-2-pyrimidinyl]amino}phenylbenzenesulfonamide (VIII):
[0045] Dissolve 13.5 g (0.05 mol) of the above-mentioned 4-bromomethylbenzenesulfonyl chloride (VII) in 100 ml CH 2 Cl 2 middle. 50ml CH containing 4-methyl-3-[4-(3-pyridyl)-2-pyrimidinyl]aminoaniline (VII) 6.1g (0.025mol) and triethylamine (5ml) was added dropwise under stirring 2 Cl 2solution. After the dropwise addition was completed, the mixture was heated to reflux for 12 hours. After the reaction was completed, cool to room temperature, a yellow solid precipitated out, the solvent was evaporated under reduced pressure, the residue was dissolved in methanol and mixed with silica gel, subjected to petroleum ether-ether (5:1) silica gel column chromatography, and the eluate was recrystallized from ethanol to obtain the intermediate 15 g of 4-bromomethyl-N-{4-methyl-3-[4-(3-pyridyl)-2-pyrimidinyl]amino}phenylbenzenes...
Embodiment 3
[0046] Embodiment 3. Preparation of one of the compounds of the present invention:
[0047] 4-[4-methyl-1-piperazinyl]methyl-N-{4-methyl-3-[4-(3-pyridyl)-2-pyrimidinyl]amino}phenylbenzenesulfonamide ( Compound code number 100504, L group is N-methylpiperazinyl)
[0048] After 0.51g (0.001mol) of the intermediate compound (VIII) prepared in Example 2 was dissolved in 15ml absolute absolute ethanol, it was added dropwise to 45ml absolute alcohol containing 0.4g (0.004mol, Shanghai Reagent Company) of nitrogen-methylpiperazine. In absolute ethanol solution, heat to reflux, use thin layer chromatography (TLC) to track and judge the reaction end point, and the reaction is complete in about 20 hours. The reaction solution was concentrated, the residue was dissolved in methanol and mixed with silica gel, the crude product was obtained by column chromatography of petroleum ether-ethyl acetate (5:1), and 0.45 g of the product was obtained by ethanol recrystallization, melting point: 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com