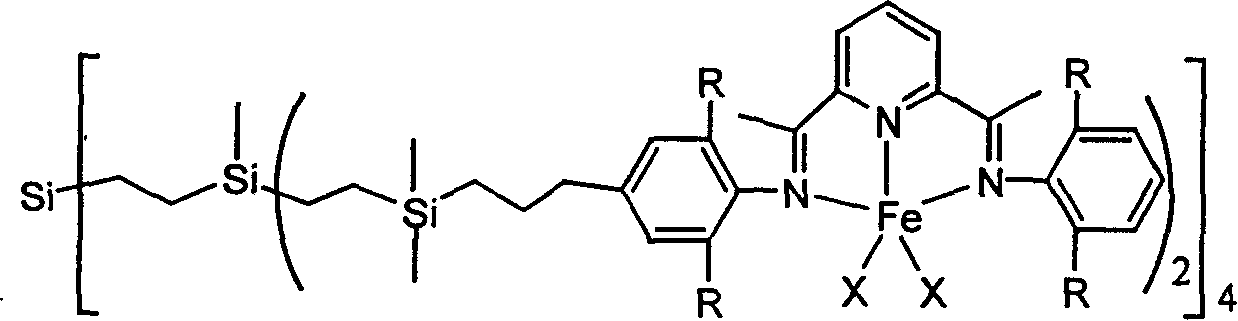
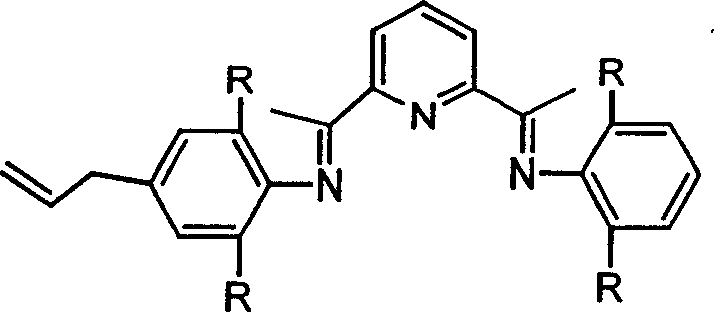
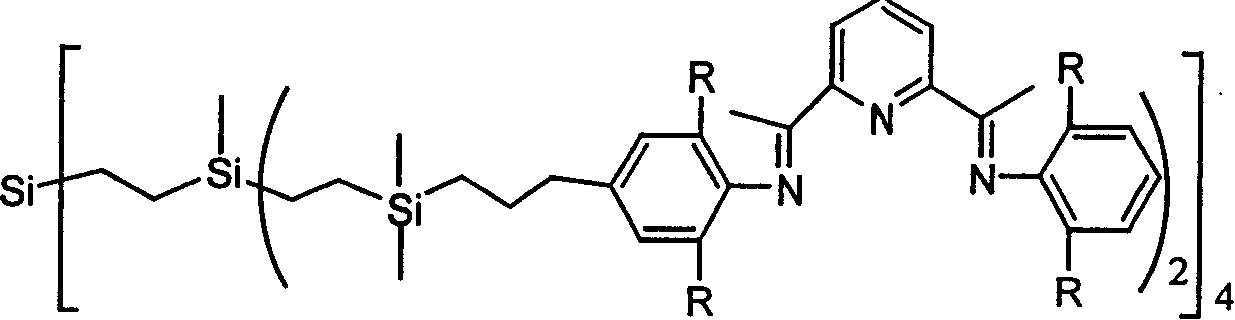
Tree polynuclear pyridine diimine iron catalyst precursor for olefine polymerization and preparation method thereof
A technology of nuclear pyridine bis-imine and pyridine bis-imine, which is applied in the field of olefin polymerization catalyst precursors, can solve the problems of slow reaction speed and catalyst embedding, and achieve the effects of stable properties and convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 100ml reaction flask, add 0.754ml of 2,6-diisopropylaniline, 0.652g of 2,6-diacetylpyridine, dropwise add 1ml of formic acid as a catalyst, stir at room temperature for 24 hours under nitrogen, a yellow precipitate appears, after filtration , the obtained yellow powder was washed 3 times with cold methanol to obtain the monoimine compound, then the monoimine compound was dissolved in 30ml of isopropanol, a small amount of dichloromethane was added to make it completely dissolved, and then an excess of 4-allyl- 2,6-Diisopropylaniline, 1 mL of formic acid was used as a catalyst, and a yellow precipitate appeared when heated under reflux for 24 hours. After filtration, the obtained yellow powder was washed three times with cold isopropanol and dried in vacuo to obtain 1.3 g of pyridinebisimine compound, Yield 77%. 1 HNMR (CDCl 3 ): δ8.46-8.5 (br, 2H, Py-Hm), 7.94 (br, 1H, Py-Hp), 7.17 (d, 2H, Ph-Hm), 7.11 (t, 3H, Ph-Hp), 6.98 (s, 2H, Ph-Hm), 6.05 (br, 1H, -CH=C), 5....
Embodiment 2
[0031] Use 2,6-dimethylaniline 0.54g (4mmol) to replace the 2,6-diisopropylaniline in Example 1, and a slightly excess 4-allyl-2,6-dimethylaniline to replace the example 4-allyl-2,6-diisopropylaniline in 1, and other operations were the same as those in Example 1, to obtain 1.2 g of a pyridinebisimine compound with a yield of 75%.
Embodiment 3
[0033] In a 100mL reaction flask, 1.36g (10mmol) of tetravinylsilicon and 7.5g (80mmol) of dimethylchlorosilane were added, 1mg of chloroplatinic acid was added in an ice-water bath, stirred for 2 hours, and then reacted at room temperature for 8 hours, and then the solvent was removed in vacuo With excess chlorosilane, 4.9 g of tetrakis (chlorosilane) compound was obtained with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com