Method for preparing aliphatic polyester
An aliphatic polyester and cyclic ester technology, which is applied in the field of polymer materials, can solve the problems of tin's poor health of children, weak polymer structure regulation ability, and high polymerization temperature, so as to improve quality, application safety, and polymer molecular weight. The effect of narrow distribution and low polymerization temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] In the preparation method of aliphatic polyester provided by the present invention, the cyclic ester monomer is selected from: glycolide, lactide, β-propiolactone, β-butyrolactone, γ-butyrolactone, γ -Any one of valerolactone, δ-valerolactone, ε-caprolactone, 1,4-dioxan-2-one, 1,5-dioxoheptan-2-one or a mixture thereof .
[0019] In the preparation method of the aliphatic polyester provided by the present invention, the cyclic ester monomer is preferably selected from glycolide, lactide, β-butyrolactone, δ-valerolactone, ε-caprolactone, 1 , any one of 4-dioxan-2-one, 1,5-dioxoheptan-2-one or a mixture thereof.
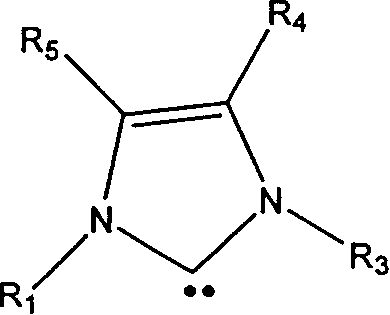
[0020] In the preparation method of aliphatic polyester provided by the present invention, the general structural formula of described metal-free N-heterocyclic carbene catalyst is:
[0021]
[0022] Among them, R 1 , R 3 selected from H atom, C 1 ~C 20 Alkyl, C 3 ~C 20 Branched chain alkyl, C 5 ~C 12 Cycloalkyl, Substituted Cycloalkyl, C 1 ~C 6 A...
Embodiment 1
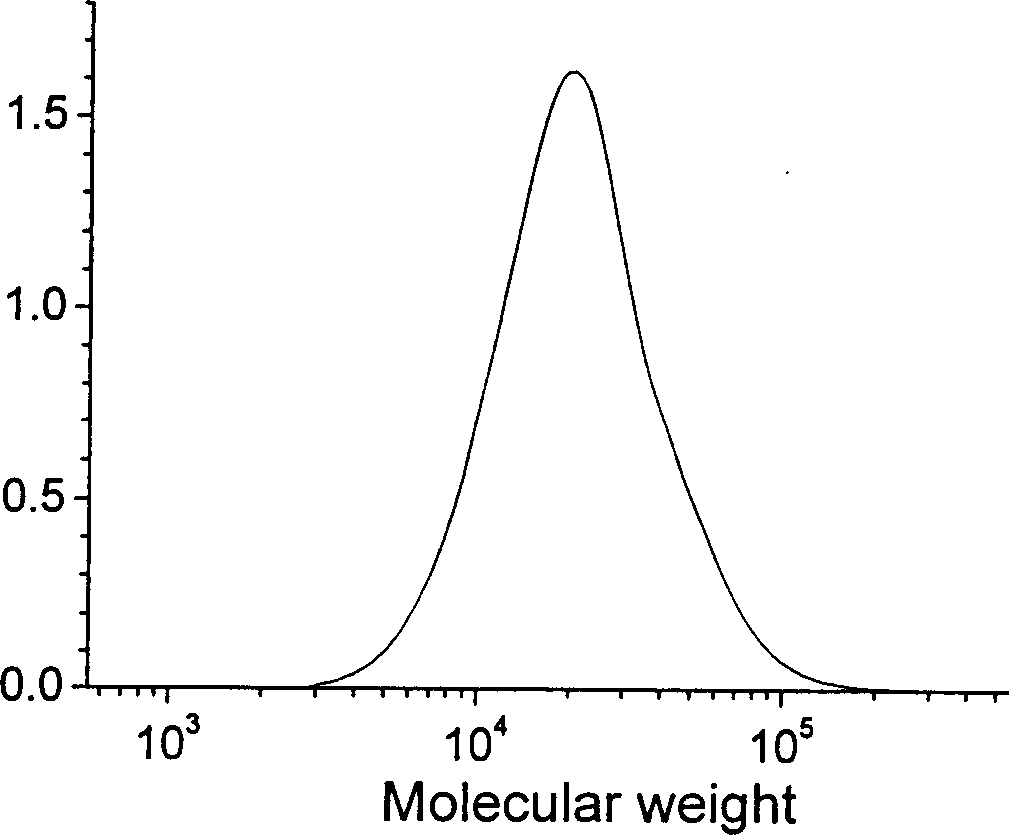
[0046] Under anhydrous and oxygen-free conditions protected by high-purity nitrogen, mix 26 mg (0.1 mmol) of 1,3-dibutylimidazolium bromide (bbimBr) and potassium tert-butoxide 10 mg (0.09 mmol) uniformly, and fully React for 20 minutes to generate 1,3-di-n-butylimidazolcarbene (bbim) catalyst, then add monomer ε-caprolactone 1ml (9.4mmol) and initiator benzyl alcohol 10.8mg (0.1mmol), stir to make it mix uniform. After reacting at 25° C. for 30 minutes, a terminator water was added to terminate the reaction. 10 ml of tetrahydrofuran was added to dissolve the obtained polymer, and after precipitation, filtration and drying, polyε-caprolactone was obtained. The monomer conversion rate was 99.2%. The number average molecular weight measured by GPC is 9960, and the polydispersity index is 1.39. The molecular weight distribution curve of polyε-caprolactone is shown in figure 1 .
Embodiment 2
[0048] Under anhydrous and oxygen-free conditions protected by high-purity nitrogen, 10 mg (0.09 mmol) of potassium tert-butoxide was dissolved in 2 ml of tetrahydrofuran, and then 26 mg of substituted imidazolium salt 1,3-dibutylimidazolium bromide (bbimBr) was added ( 0.1 mmol), stirred to make it evenly mixed, reacted at 25° C. for 20 minutes, and filtered to obtain 1,3-di-n-butylimidazolecarbene (bbim) catalyst solution. Then, 1 ml (9.4 mmol) of monomer ε-caprolactone, 5 ml of tetrahydrofuran, and 10.8 mg (0.1 mmol) of benzyl alcohol were added, and stirred to make them evenly mixed. After reacting at 25° C. for 30 minutes, a terminator water was added to terminate the reaction. The obtained polymer solution is precipitated, filtered and dried to obtain polyε-caprolactone. The monomer conversion rate was 95.3%. The number average molecular weight measured by GPC is 8960, and the polydispersity index is 1.34.
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com