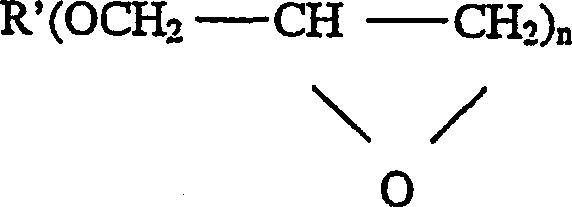
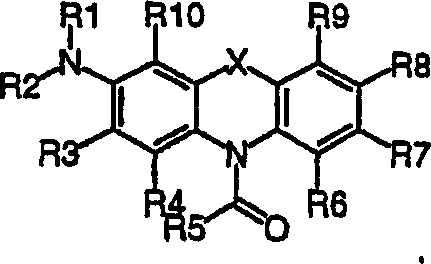
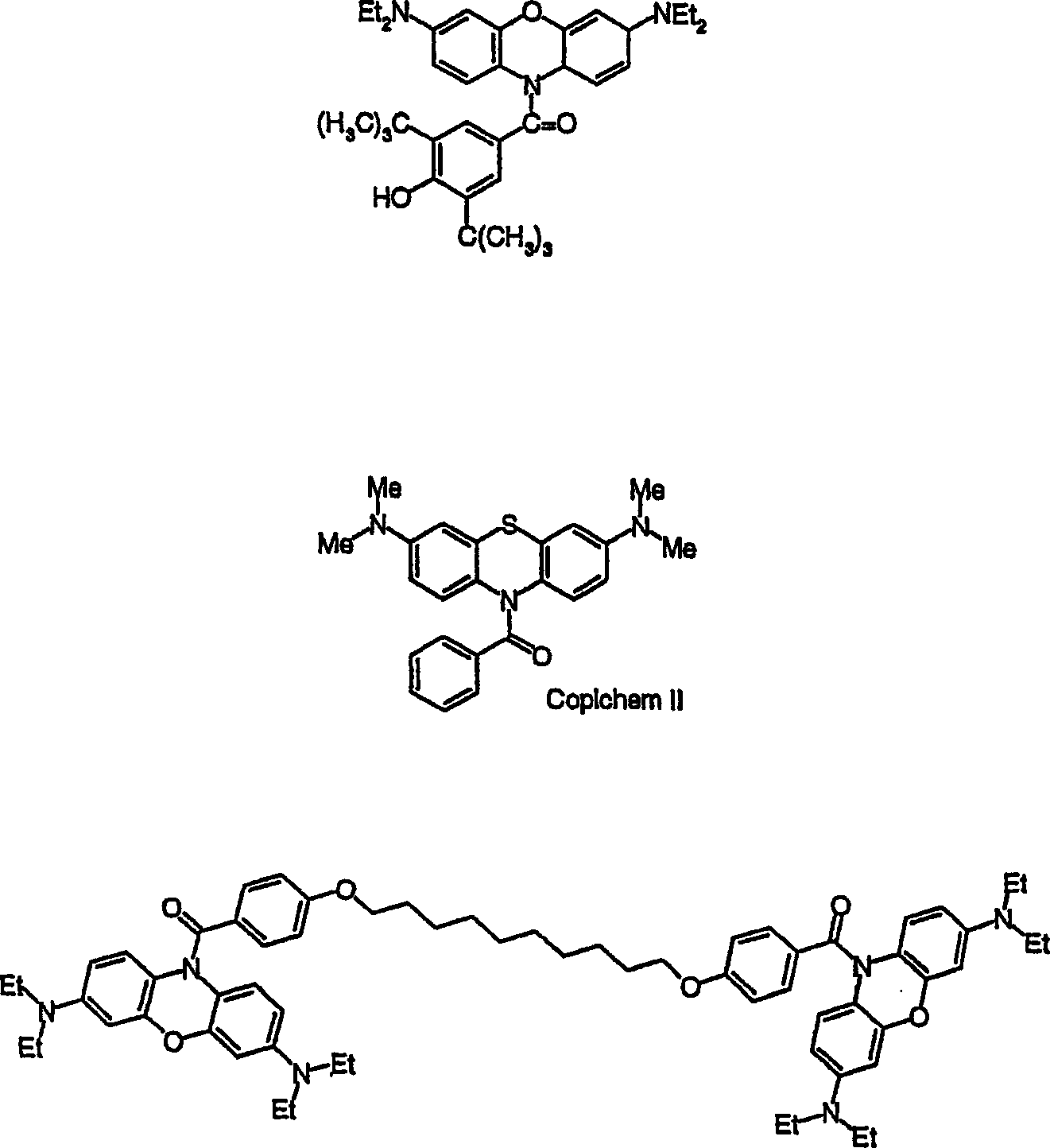
Multiphoton photosensitization system
A multi-photon and photosensitizer technology, which is applied to photosensitive materials, optics, and optical components used in optomechanical equipment, can solve problems such as low photosensitivity, slow writing speed, and high laser power, and achieve enhanced multi-photon photosensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0172] Multiphoton-Initiated Free Radical Polymerization
[0173] An up-converting inorganic phosphor ((Y 0.86 Yb 0.08 Er 0.06 )O 2 S, Green UC-3 (purchased from AlliedSignal Inc., Seeize, Germany) was used as a multiphoton photosensitizer to polymerize pentaerythritol tetraacrylate (purchased from Sartomer Co., Exton, PA as SR295) to form a hydrogel. Light output from a 980 nm continuous wave laser diode (80 mW on a 9 mm head with feedback photodiode, purchased from Semiconductor Laser International of Binghamton, NY, with an average operating power of 50 mW) was passed through a focusing lens and used to initiate polymerization. The diameter of the focused spot was measured to be 50 micrometers. Therefore, the energy density used to initiate polymerization with the phosphor is only 2×10 3 W / cm 2 . 20 g of SR295 was dissolved in a mixture of 100 g of acrylonitrile and 25 g of water to form a stock solution of the tetraacrylate. To 8 g of the stock solution of the tetr...
Embodiment 2
[0181] Multiphoton-Initiated Cationic Polymerization
[0182] will Epon TM SU-8 (104.25 g, bisphenol A novolac epoxy resin, available from Resolution Performance Products, Houston, TX) was dissolved in propylene glycol methyl ether acrylate (PGMEA) (48.5 g, available from Aldrich, Milwaukee, WI). 5,7-Diiodo-3-butoxy-6-fluorone (H-Nu 470B, purchased from Spectra Group, Ltd. Maumee, OH) (68 mg), 69 mg hexafluoroantimonic acid (4-(2- Hydroxytetradecyloxy)phenyl)phenyliodonium (available as CD1012 from Sartomer Co., Exton, PA) and 5 mg ethyl 4-dimethylaminobenzoate (EDDMAB, available from Aldrich, Milwaukee, WI) were dissolved In a minimal amount of tetrahydrofuran (ca. 1 g) and adding this to 10 g of the above SU-8 / PGMEA solution with stirring, to the resulting mixture was added the up-converting inorganic phosphor (Y 0.8 Yb 0.2 T m 0.00075 )F 3 (34 mg) (an up-converting phosphor of this type is commercially available from Allied Signal Inc., Seeize, Germany) and stirred to...
Embodiment 3
[0185] Writing images via multiphoton-induced radical polymerization
[0186] 90 g of polymethyl methacrylate (PMMA, molecular weight 120,000, purchased from Aldrich, Milwaukee, WI) and 368 g of 1,2-dichloroethane were mixed, and the mixture was stirred overnight to obtain polymethyl methacrylate ( PMMA) stock solution in dichloroethane. The resulting stock solution (15 g) was mixed with 3.5 g of tris(2-hydroxyethyl)isocyanurate triacrylate (available as SR368 from Sartomer Co., Exton, PA) and 3.5 g of alkoxylated trifunctional Acrylates (available as SR 9008 from Sartomer Co., Exton, PA) were mixed, and the resulting mixture was heated and stirred to obtain an acrylate stock solution. 13 mg rose bengal, 13.6 mg 4-(2-hydroxytetradecyloxy)phenylphenyliodonium hexafluoroantimonate (available as CD1012 from Sartomer Co., Exton, PA) and 14.3 mg n-butyltriphenyl Tetramethylammonium borate (available as CGI 437 from Ciba Specialties Chemicals Corp., Tarrytown, NY) was mixed with 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com