Nanometer granule prepn of bufanin albumin and its prepn process
A technology of albumin nanoparticles and bufalin, which is applied in the field of medicine, can solve the problems of organic solvent residues, limited reliability, and difficult purification of nanoparticles, and achieve less organic solvent residues, lower toxic and side effects, and strong operability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1. Preparation of bufolin albumin nanoparticle preparation
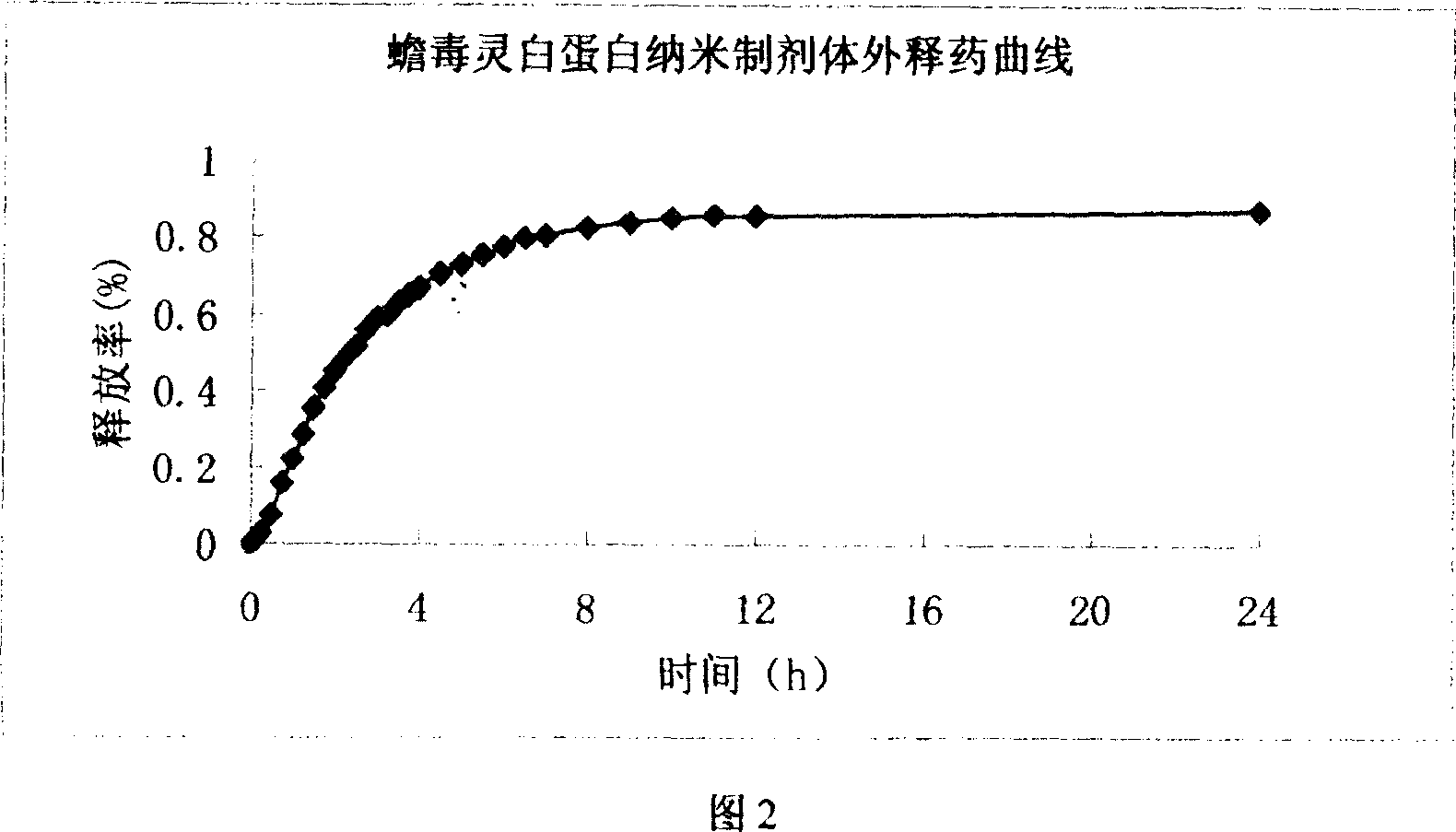
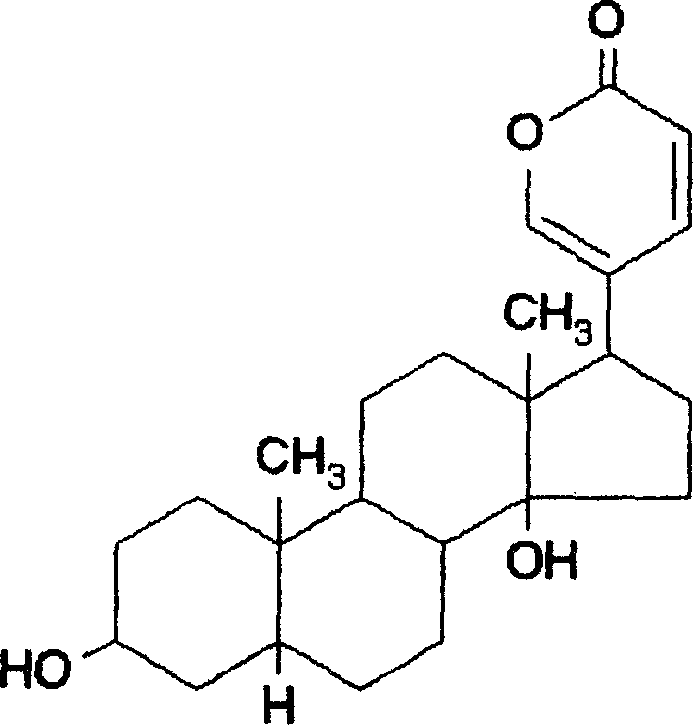
[0032] Dissolve 50mg of albumin in 2ml of water, dissolve 5mg of bufalin in 1ml of ethanol, add bufalin alcohol solution into the albumin aqueous solution, control the pH value between 5-7, stir at 0.5ml / Continue to add 3ml of ethanol at a speed of min until an emulsion colloidal suspension is formed. Add 27μl of cross-linking agent glutaraldehyde under stirring, and stir continuously for 24 hours to promote the cross-linking and solidification of nanoparticles. Gently heat and evaporate under reduced pressure to remove organic solvents such as ethanol. Freeze-dry to obtain the bufalin nanoparticle preparation of the present invention.
Embodiment 2
[0033] Example 2 Preparation of bufolin albumin nanoparticle preparation
[0034] Dissolve 100mg of albumin in 4ml of water, dissolve 20mg of bufalin in 2ml of ethanol solution, add bufalin alcohol solution into the albumin aqueous solution, control the pH value between 5-7, and continuously add an appropriate amount of dehydrating agent under stirring Acetone, to form a milky colloidal suspension, add an appropriate amount of cross-linking agent methyl polyethylene-dextran under stirring to form a milky white opaque liquid, continue stirring for 26 hours to promote cross-linking and solidification of nanoparticles, evaporate under low heat and decompression, remove organic solvents such as ethanol and acetone, and freeze-dried to obtain the bufalin nanoparticle preparation of the present invention.
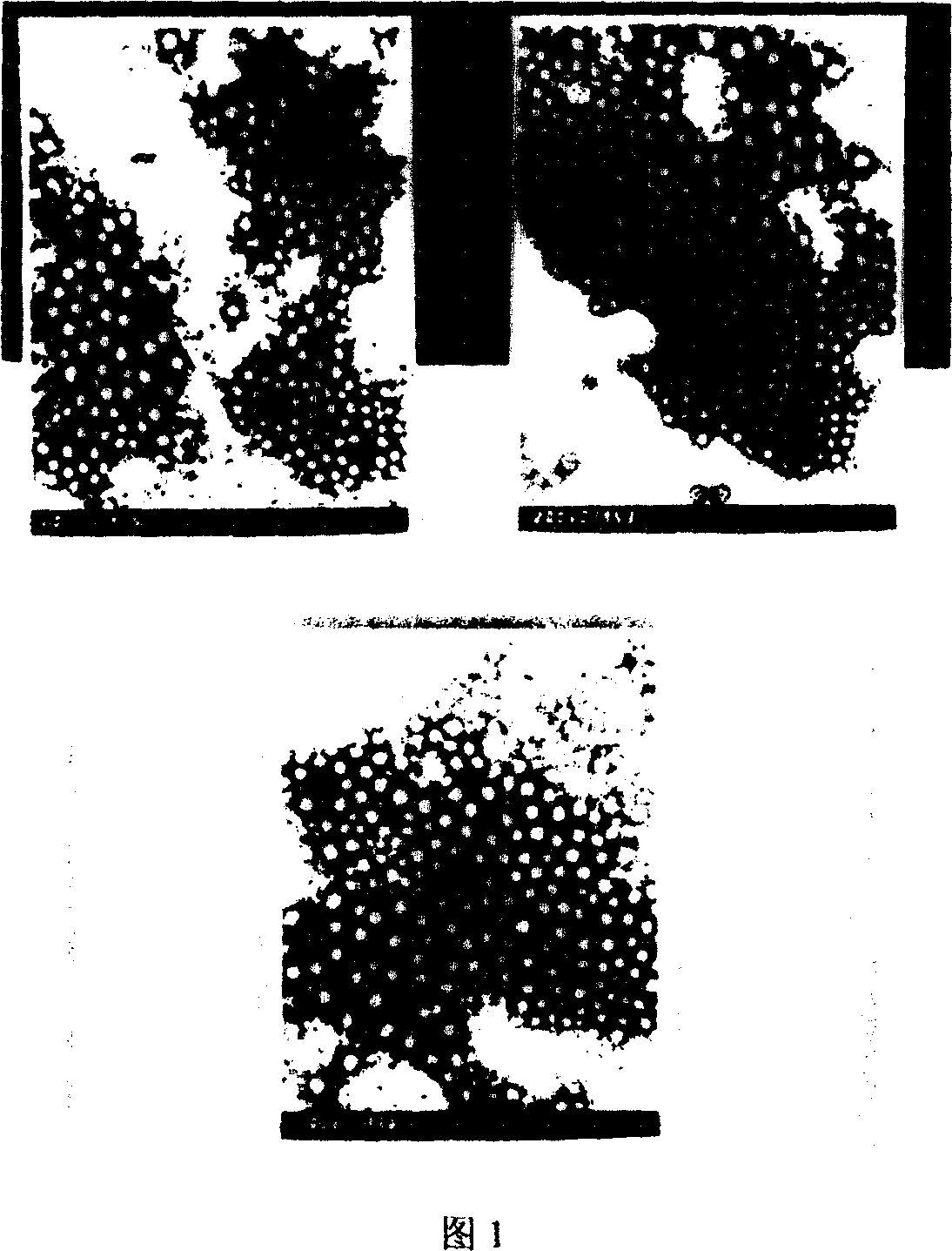
[0035] Observation of appearance and morphology: adopt tungsten phosphate negative staining method, observe the appearance and morphology of bufalin nanoparticles prepared by the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com