Layered lithium metal oxides free of localized cubic spinel-like structural phases and methods of making same
A spinel structure, layered crystal structure technology, applied in the direction of alkali metal oxide/hydroxide, alkali metal compound, lithium oxide;/hydroxide, etc., can solve problems such as battery cycle problems, achieve good Structural stability, effect of uniform electrochemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
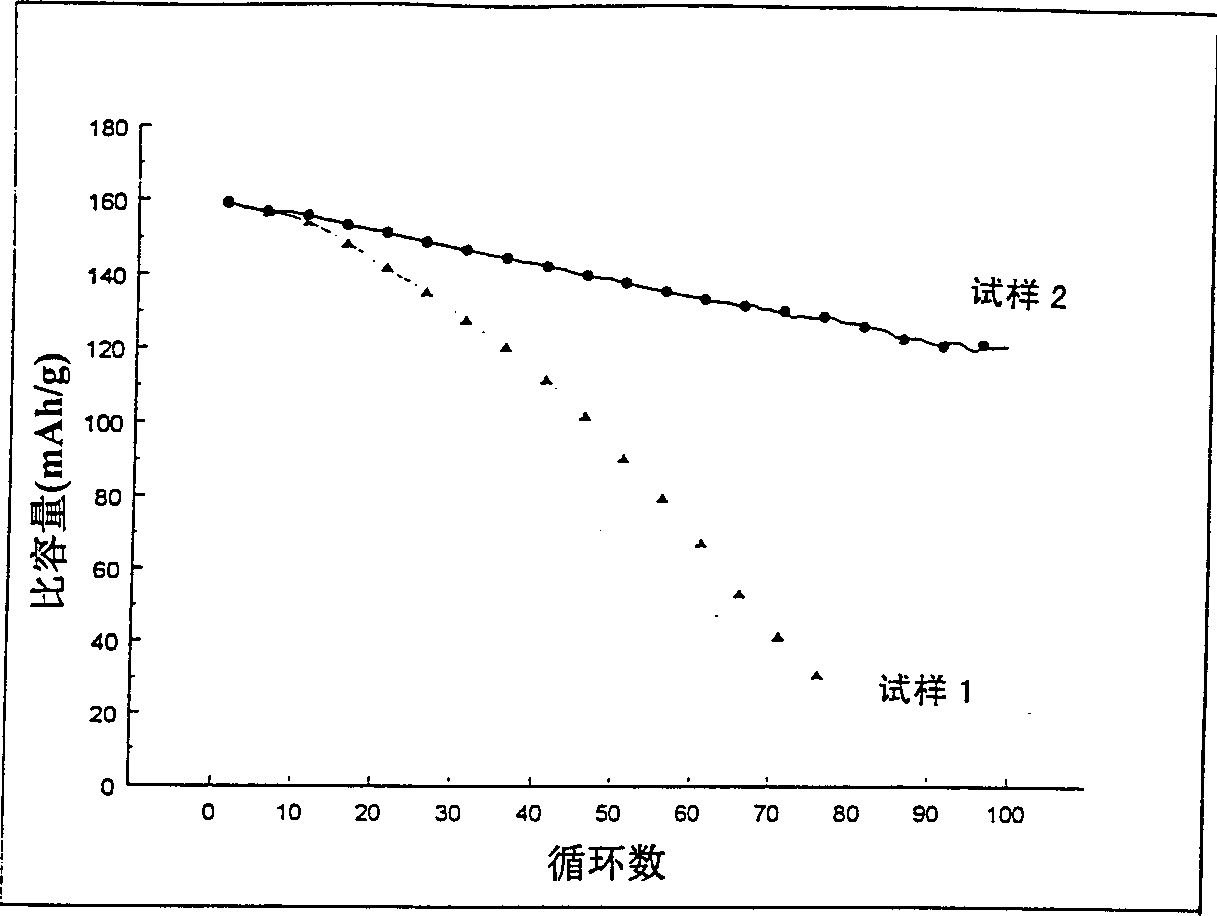
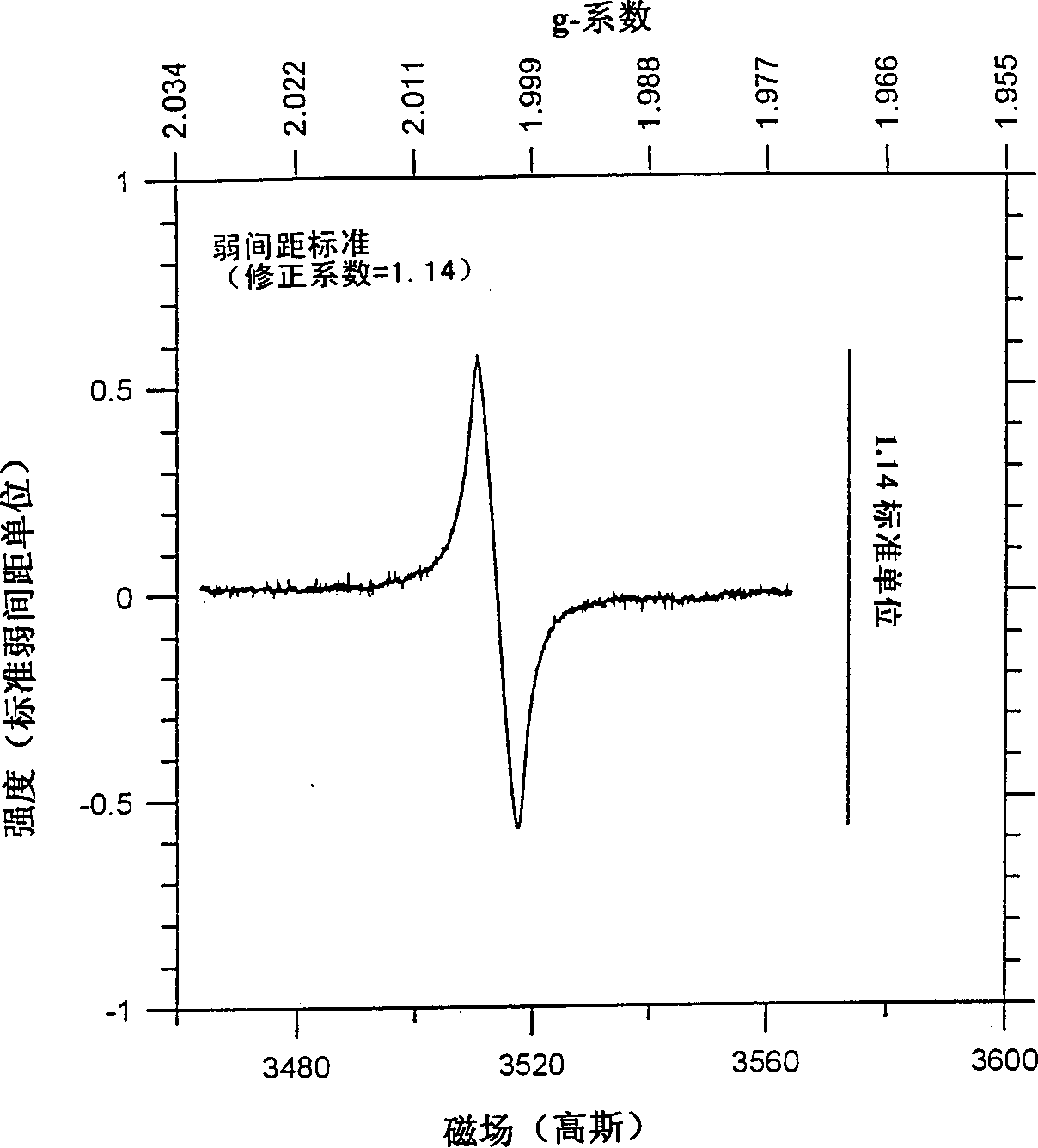
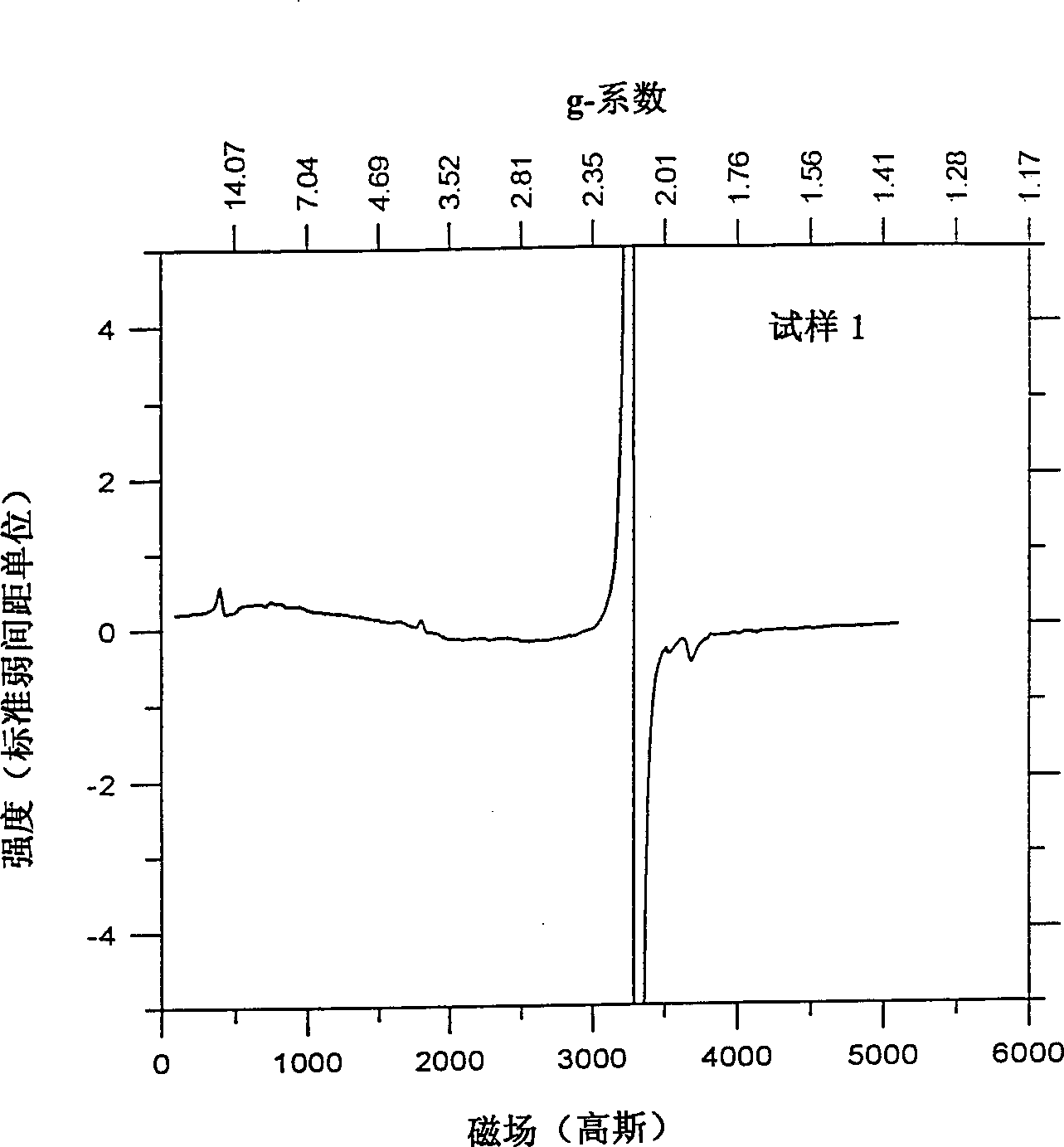
[0042] Industrial LiCoO 2 The sample (sample 1) was heated to 950°C for 1 hour, then rapidly cooled by removing the sample directly from the hot zone and spreading the sample flat on a stainless steel pan at room temperature. The cooling time from 950°C to room temperature is estimated to be about 10 minutes. The sample 1 and the fast cooling sample (sample 2) were used as positive electrode materials for different electrochemical cells, and each battery used lithium metal as the negative electrode coin cell structure. NRC2325 coin cell components and Celgard 3501 separators were used. The electrolyte is 1M LiPE 6 A 50:50 mixture of ethylene carbonate and dimethyl carbonate. The positive electrode contains 85% active material (by weight), 10% super S TM Carbon black and 5% polyvinylidene fluoride (PVDF) as polymer binder were coated on aluminum foil. The cycle test is carried out between 3.0V and 4.3V, and a constant current of C / 3 (3 hours for complete charge and dischar...
Embodiment 2
[0049] Mixed stoichiometric Li 2 CO 3 and Co 3 o 4 , then heated from room temperature to 950 °C at a rate of 3.75 °C / min, held at 950 °C for 5 hours, and then cooled to room temperature at a rate of about 3.7 °C / min (the total cooling time was slightly longer than 4 hours). The obtained compound was sample 3.
[0050] Mixed stoichiometric Li 2 CO 3 and Co 3 o 4 , then heated from room temperature to 950 °C at a rate of 3.75 °C / min, maintained at 950 °C for 5 hours, and then cooled to room temperature at a rate of about 8 °C / min (the entire cooling time was only less than 2 hours). The obtained compound was sample 4.
[0051] Sample 3 and sample 4 were subjected to cycle detection according to the method of Example 1. Figure 7 The cycle characteristics of sample 3 and sample 4 were compared. Such as Figure 7 As shown, the cycle performance of sample 4 prepared according to the present invention is better than that of sample 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com