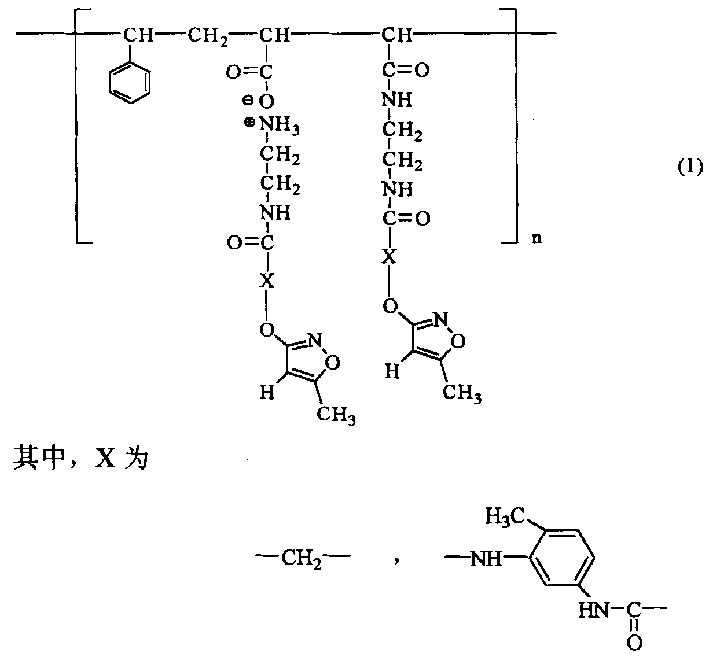Polymer chemical slow release fungicide contg hymexazol active component
A polymer type, fungicide technology, applied in biocides, animal repellents, plant growth regulators, etc., can solve problems such as uneconomical, and achieve the effects of less application times, longer duration of effect, and stable pharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Example 1 Synthesis of 2-[3'-(5'-methylisoxazolyl)]oxyacetic acid
[0030]Drop into 15 grams (0.1500 moles) of 3-hydroxyl-5-methyl-isoxazole (that is: hymexazol) and 50 ml of water, start stirring, heat up to 60°C, add 50% sodium hydroxide solution to adjust the pH to 8-9, feed nitrogen, heat up to 95°C, add dropwise 21.5 g of chloroacetic acid (0.2250 moles), 9.5 g of hydrogen Sodium oxide (0.2260 mole) and the solution that 40 milliliters of water are made into, add in 20~30 minutes, keep warm and reflux for 4~5 hours to the end of reaction. Then cool down to 75-80°C, add 10% hydrochloric acid dropwise to adjust the pH value to 1-2, stir for 30 minutes, add 100 ml of toluene, continue stirring for 30 minutes, pour it into a separatory funnel while it is hot, and separate the water phase. After distilling off the toluene, 11.3 g of a light yellow solid was obtained, which was the product, with a purity of 95% and a yield of 48%. After methanol recrystallization, the ...
example 2
[0031] Example 2 Synthesis of 3-(3'-isocyanato-4'-methylanilinocarbonyl)oxy-5-methyl-isoxazole
[0032] Drop into 36.2 grams (0.2000 moles) of toluene-2,4-diisocyanate, catalyst and 50 milliliters of 1,2-dichloro Ethane, start stirring, feed nitrogen, heat up to 75-80°C, add dropwise 10 g (0.1000 mol) of 3-hydroxy-5-methyl-isoxazole (ie: hymexazol) and 50 ml of 1, Add the solution made of 2-dichloroethane in 45-60 minutes, keep warm and reflux for 9 hours to the end of the reaction. Cool down to room temperature, let stand for 24 hours, filter, rinse the filter cake with 10 ml of dry 1,2-dichloroethane, and vacuum dry the filter cake to obtain 22.3 g of white powder with a purity of 90.2% and a yield of 82%. After recrystallization from dried chloroform, white needle-like crystals were obtained, with a purity of 99.5%, and a melting point of 129-131°C; 1 H NMR (CDCl 3 )δ H : 2.27 (3H, s, 5-CH 3 ), 2.36 (3H, s, 4'-CH 3 ), 5.56(1H, s, 4-H), 6.99~7.91(3H, m, 2', 5', 6'-H), ...
example 3
[0033] Example 3 Synthesis of styrene-maleic anhydride alternating copolymer
[0034] 10.5 grams (0.1000 moles) of styrene, 9.9 grams (0.1000 moles) of maleic anhydride and 120 milliliters of toluene were dropped into a 250 milliliter three-neck flask equipped with stirring, a thermometer and a reflux condenser, and the temperature was raised to 75 ~80°C, add dropwise a solution made of 0.1g of benzoyl peroxide and 10ml of toluene, add it in about 15 minutes, keep it warm at 85~90°C for 4~5 hours, cool down to room temperature, filter, and use 50 The filter cake was rinsed three times with milliliter of toluene, and after the filter cake was dried, 20.0 g of polymer (I) was obtained as a white powder, with a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com