Absorbing compounds for spin-on glass anti-reflective coatings for photolithography
A technology for spinning glass and compounds, which is applied in the field of light-absorbing spinning glass materials and absorbing compounds, and can solve problems such as increasing processing complexity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
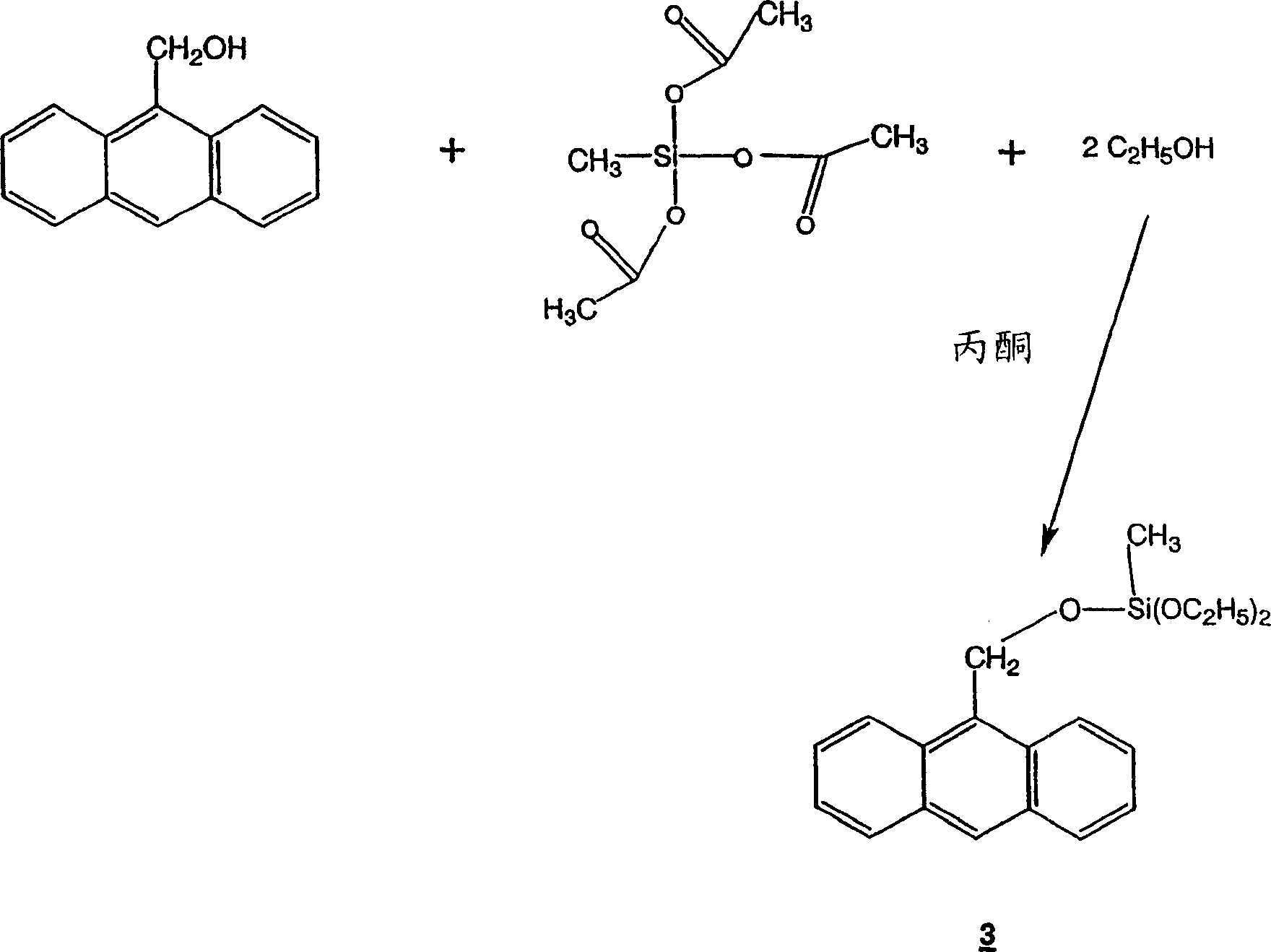
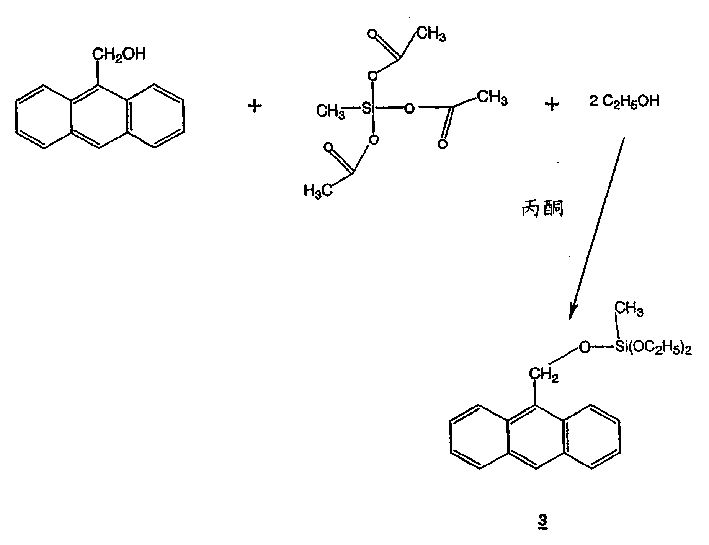
[0030] Synthesis of 9-Anthracenemethoxy-methyldiethoxysilane
[0031] In a 3-liter flask, mix 92.37 g (0.419 mol) methyltriacetoxysilane (MTAS), 87.36 g (0.419 mol) 9-anthracenemethanol, 38.56 g (0.839 mol) ethanol, 595.51 g (10.20 mol) acetone . The solution was stirred under nitrogen atmosphere for 7 days. The solution was degassed to remove the acetic acid by-product.
Embodiment 2
[0033] Synthesis of Absorbent SOG Containing 9-Anthracenemethoxymethyldiethoxysilane
[0034] In a 1-liter flask, 297 g (4.798 mol) of isopropanol, 148 g (2.55 gmol) of acetone, 123 g (0.593 mol) of TEOS, 77 g (0.432 mol) of MTEOS, 200 g of 9-anthracenemethoxy -Methyldiethoxysilane, produced in Example 1, 2.61 grams (0.009) mol rosenic acid, 10 grams (0.024mol) 2-hydroxy-4 (3-triethoxysilyl propoxy) - Benzophenone, 0.09 grams (0.0004 mol) of 2,6-dihydroxyanthraquinone, 0.6 grams of 1.0 M nitric acid and 72 grams (3.716 mol) of deionized water were mixed. The flask was refluxed for 4 hours. 43 grams (0.590 mol) of butanol were added to the solution. The solution was filtered. The solution was dispensed, followed by thickness spinning at 3000 rpm for 20 seconds and baking at 80°C and 180°C for 1 minute respectively. Optical properties were measured with a N & K Technology Model 1200 analyzer. The film thickness was 2801 angstroms. At 248 nm, the refractive index (n) is 1.4...
Embodiment 3
[0036] Synthesis of 9-Anthracenemethoxy-triethoxysilane
[0037] In a 3 liter flask, 110.73 grams (0.419 mol) of tetraacetoxysilane (TAS), 87.36 grams (0.419 mol) of 9-anthracenemethanol, 57.98 grams (1.2585 mol) of ethanol, 595.51 grams (10.20 mol) of acetone were mixed. The solution was stirred under nitrogen atmosphere for 7 days. The solution was degassed to remove the acetic acid by-product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com