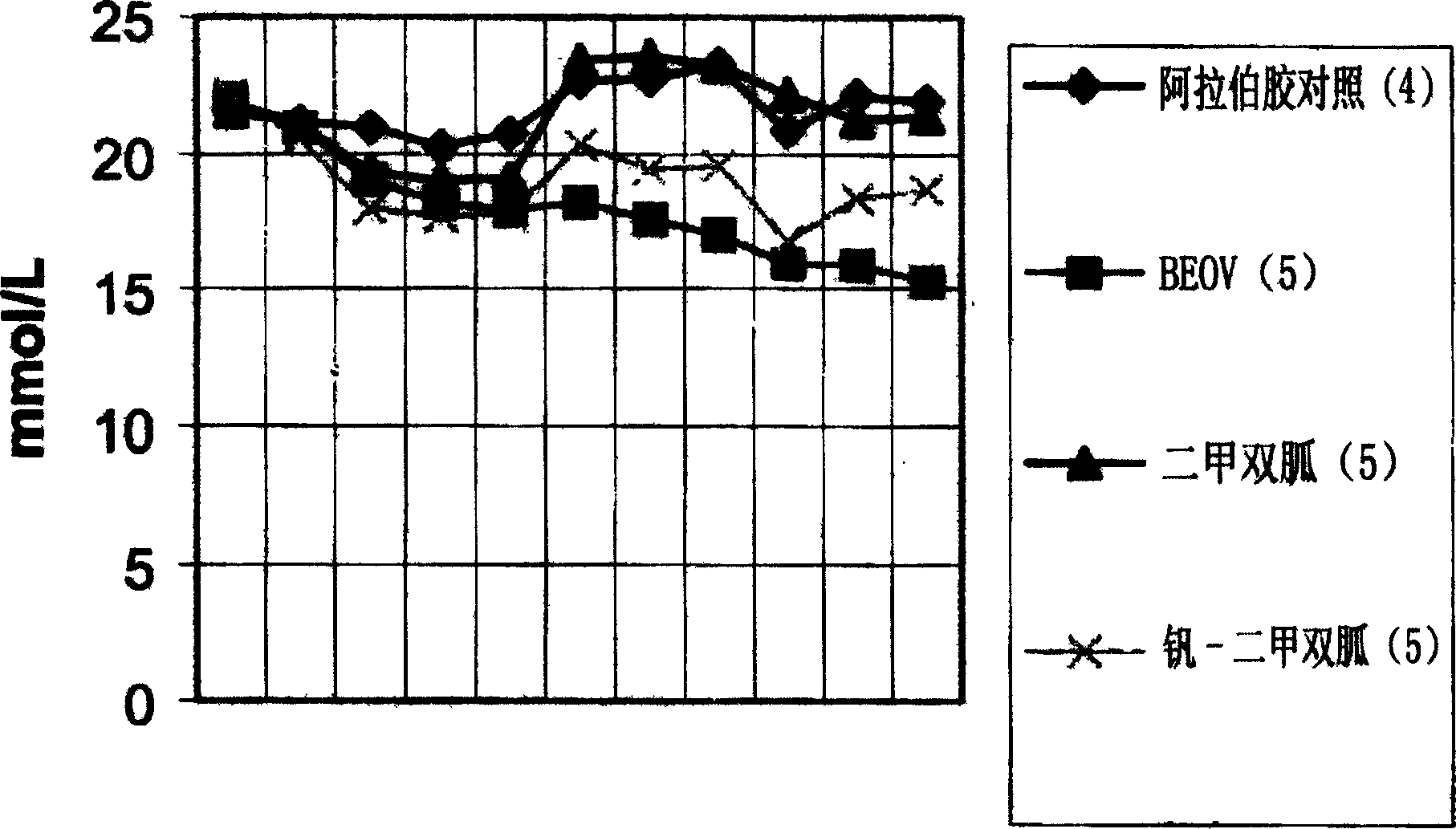
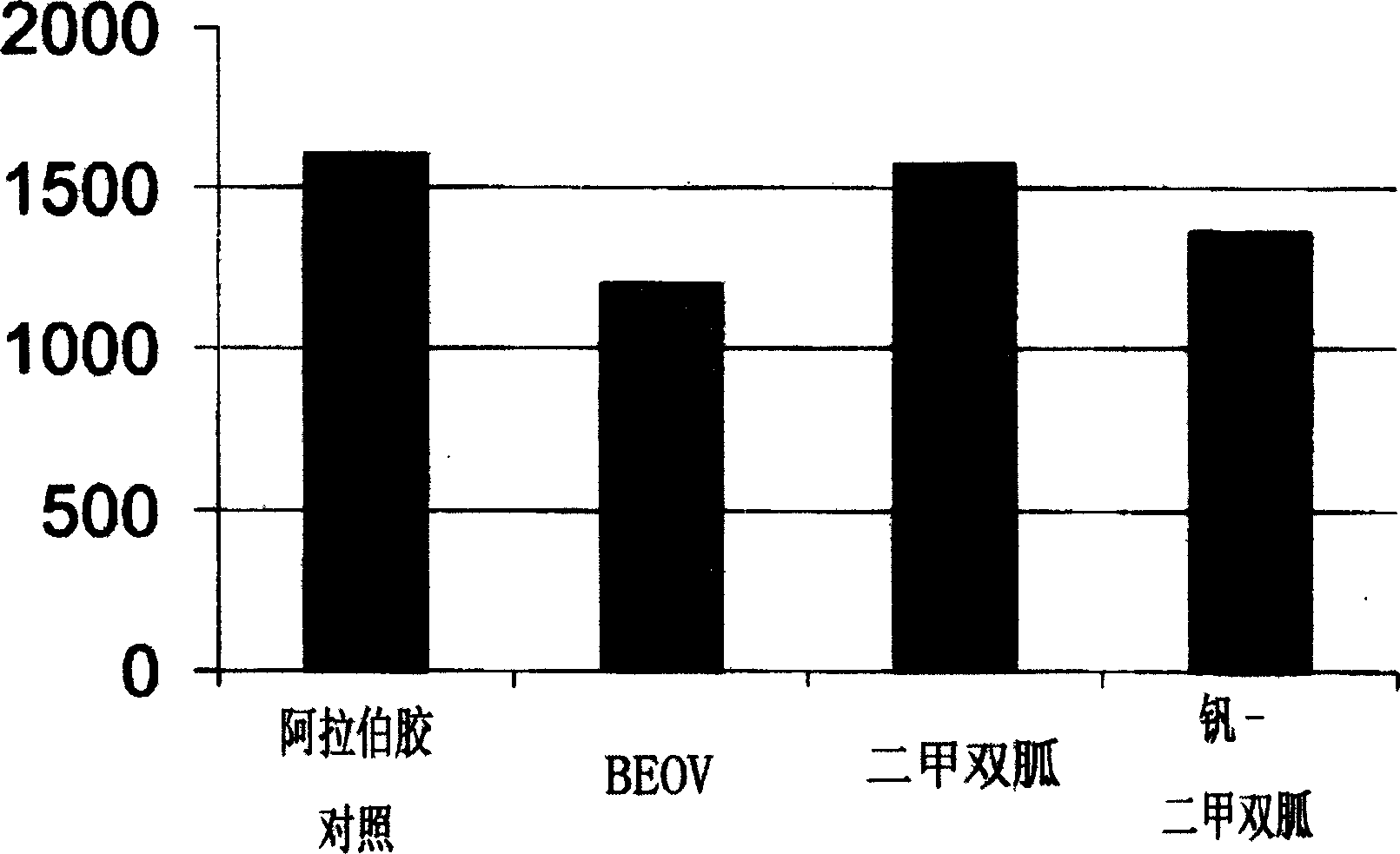
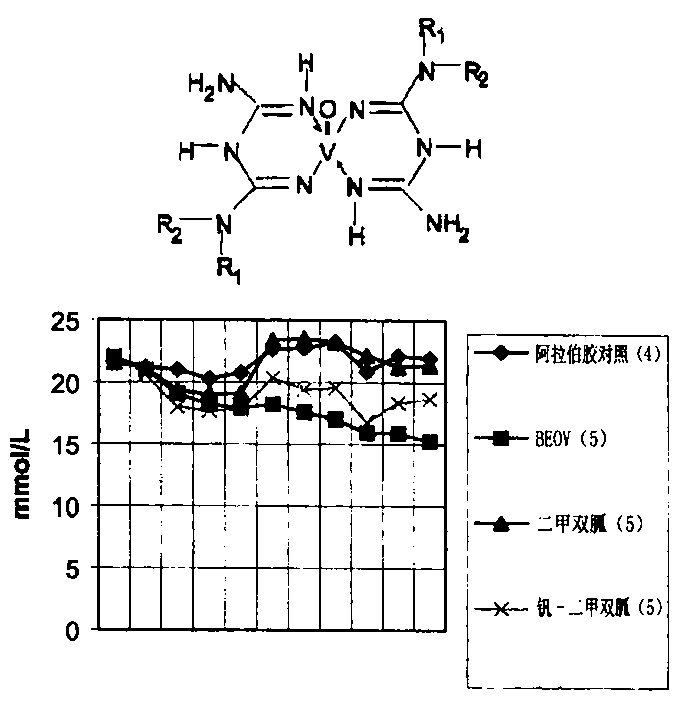
Biquanide vanadium complex plaster preparation for curing diabetes and its application
A technology of diabetes drugs and complexes, which is applied in the direction of drug combinations, active ingredients of heavy metal compounds, metabolic diseases, etc., can solve the problems of unable to maintain blood drug concentration, low bioavailability, obvious side effects, etc., and achieve individual differences Small, avoid oral administration, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] This application provides the preparation of the following patch as an example, but it is not limited to the scope mentioned in the application documents, and can be fully extended to the known film controlled release type, matrix controlled release type, and micro reservoir dissolution controlled release type Or dispersed patches in adhesives, the applicant believes that all patches and their equivalents that contain the main active ingredients pointed out in the present invention and are used for the treatment of non-insulin-dependent diabetes mellitus (NIDDM) and related diseases should belong to protection scope of this patent.
[0038] The skin covering the body surface is only 3mm thick, but it is the largest tissue in the human body. The skin is a barrier between the external environment and the body. Transdermal absorption enhancers refer to substances that can accelerate the speed of drug penetration through the skin without causing serious irritation and damag...
Embodiment 1
[0041] The selected compound is 1,1-dimethylbiguanide vanadium complex, and the vanadium in the complex is coordinated with oxygen; in this pharmaceutical preparation, in addition to the main active ingredient, the transdermal absorption accelerator used is laurocapram (Azone Azone) or menthol; the absorption and penetration aid is ethanol or propanol; the consumption of both is 3.0%; the softening agent is glycerin or polyvinyl alcohol, and the consumption is 5.0%. Except that the main active ingredients are prepared by the US patent method, the rest are all commercially available products.
[0042] The specific weight ratio is:
[0043] 1,1-Dimethylbiguanide vanadium complex 100kg, laurocapram or menthol 3kg, ethanol or propanol 3kg, glycerin or polyvinyl alcohol 5kg, essence 0.5kg.
[0044] Weigh all the materials according to the proportion (the solid is crushed into the required fineness in advance, and dried to the water content ≤ 0.5%), put it in the container and mix ...
Embodiment 2
[0053] The selected compound is 1,1-dimethylbiguanide vanadium complex, and the vanadium in the complex is coordinated with oxygen; in the pharmaceutical preparation, the transdermal absorption accelerator used in addition to the main active ingredient is laurocapram; Absorption and penetration aids are ethanol or propylene glycol; emollients are glycerin or polyvinyl alcohol.
[0054] The specific weight ratio is:
[0055] 1,1-Dimethylbiguanide vanadium complex 100kg, laurocapram 5kg, ethanol or propylene glycol 2.5kg, glycerin or polyvinyl alcohol 3kg, essence 0.5kg.
[0056] Obtain the patch of dispersion type in the adhesive agent through conventional pressure glue, paste preparation, coating, drying, cutting, packing; Remaining with embodiment 1. Example 3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com