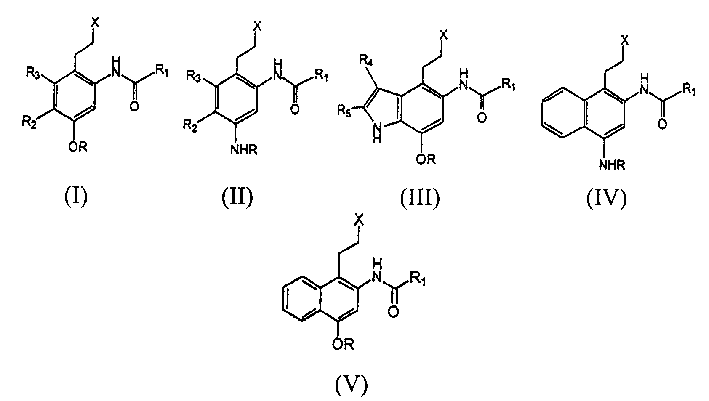
Compositions and methods of use thereof achiral analogues of CC-1065 and duocarmycins
A compound and chloride technology, which is applied to the composition of CC-1065 and the new achiral analogs of Docamex and its application field, can solve the problems of low efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 12 Preparation of 2-(2-amino-4-hydroxyphenyl)ethyl bromide and 2-(2-amino-4hydroxyphenyl)ethyl chloride
[0061] Add EtOH (45mL) and 10% NaOH (60mL) to the round bottom containing diethyl 2-(4-benzyloxy-2-nitrophenyl)malonate (4c) (2.00g, 5.17mmol) In the flask, a dark brown solution was produced. The solution was refluxed for 3 hours and passed TLC (CHCl 3 ) Check that the solution is light yellow and clear at this time. The EtOH was removed under reduced pressure to form a yellow suspension, and enough THF (30 mL) was added to produce a clear yellow solution, which was placed in an ice bath and stirred. 6M HCl (30 mL) was slowly added to the solution to reach pH 1. The light orange solution was refluxed for another 1 hour, at which time two layers formed. Collect the upper THF layer and use CHCl 3 (100 mL) The aqueous solution was extracted. The organic layers were combined and dried over anhydrous sodium sulfate. The solution was then filt...
Embodiment 2
[0068] Example 2 N-(2-(2-chloroethyl)-4-hydroxyphenyl)-1-methyl-4-(1-methyl-4-butyramidopyrrole-2-amido)pyrrole-2 -Preparation of amides and their derivatives
[0069] Add cold methanol (100mL) to N-methyl-4-(N-methyl-4-nitropyrrole-2-amide)pyrrole-2-carboxylic acid methyl ester (3.00g, 9.8mmol) and 5% Pd / C (1.2g) mixture. The reaction mixture was hydrogenated for 18 hours and passed TLC (10% MeOH / CHCl 3 )an examination. After the reduction was completed, the amine was suction filtered with Celite and a sintered funnel, the filtrate was rotary evaporated to dryness and co-evaporated twice.
[0070] Using a syringe, distilled dichloromethane (30 mL) and butyryl chloride (1.00 mL, 10.8 mmol) were added to the dropping funnel through the septum. Then dissolve the amine in anhydrous THF (75mL) and add Et 3 N (1.5 mL, 10.8 mmol). The amine mixture was stirred in an ice bath for 5 minutes, and then the butyryl chloride mixture was slowly dropped. A light peach-c...
Embodiment 3
[0075] Example 34-(2-Chloroethyl)-3-[[5-(4-bis-(2-chloroethyl)amino)benzamide]indole-2-amide]phenol and its derivatives Preparation
[0076] A suspension of 2-(2-amino-4-hydroxyphenyl)ethyl chloride (310 mg, 1.81 mmol), 10% Pd-C (233 mg) was hydrogenated in dry THF (10 mL) at room temperature and atmospheric pressure. The catalyst was removed by filtration, and the filtrate was concentrated to obtain the amine as a white solid. Add 5-nitroindole-2-carboxylic acid (372 mg, 1.81 mmol), EDCI (1.04 g, 5.42 mmol) and dry DMF (10 mL). The reaction mixture was stirred for two days under a nitrogen atmosphere at room temperature. Use Kugelrohr device (0.1mmHg, 60□) to remove the solvent, and use CHCl 3 Distribute the residue with water. The aqueous layer was extracted with ethyl acetate (twice). Dry the combined organic extracts (Na 2 SO 4 ). The brown oily residue was purified by silica gel chromatography (0->50% ethyl acetate: chloroform per 50 mL) to isolate 4-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com