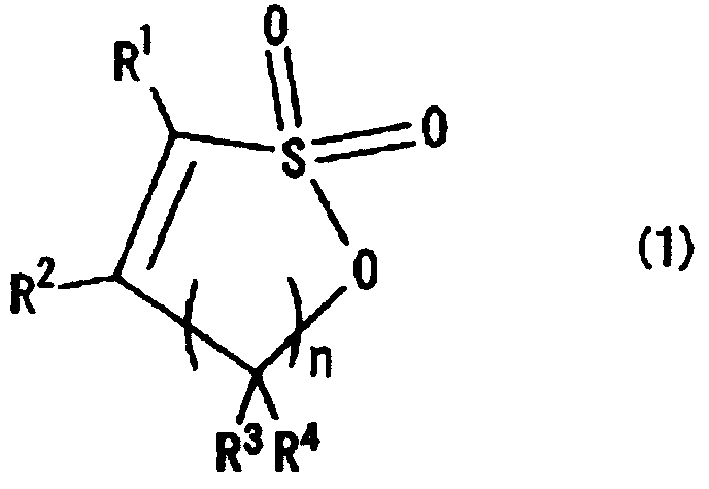
Additive for electrolytic solution, non-aqueous elecrolytic solution using said additive and secondary cell
A non-aqueous electrolyte, electrolyte technology, applied in the direction of secondary batteries, circuits, electrical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0102] Embodiment 1-6 and reference example 1
[0103] First, production of a coin-type battery will be described.
[0104] The non-aqueous electrolyte was prepared as follows. Ethylene carbonate (EC) and methyl ethyl carbonate (MEC) were mixed at a weight ratio of EC:MEC=4:5, and then LiPF as electrolyte 6 The nonaqueous electrolytic solution was prepared by dissolving in a nonaqueous solvent so that the electrolyte concentration was 1.0 mol / liter. Subsequently, 0.5% by weight of 1,3-propene sultone (Example 1), 1.0% by weight (Example 2), 1.5% by weight (Example 3), and 2.0% by weight of 1,3-propene sultone were added as additives to the non-aqueous solvent. % (embodiment 4), 2.5% by weight (embodiment 5), 3.0% by weight (embodiment 6), obtain the non-aqueous electrolytic solution of the present invention. In addition, the case where addition of an additive was omitted was made into reference example 1 (blank sample).
[0105] The negative electrode was fabricated as fol...
Embodiment 7-15
[0129] In addition to adding vinylene carbonate and adding each additive in the amount shown in Table 2 to replace 1,3-propene sultone as an additive in the preparation of the non-aqueous electrolyte in Example 1, the rest are the same as in the example 1 In the same way, a coin-type battery was produced, and its battery characteristics were measured. The results are shown in Table 2.
[0130]
[0131] From the above results, it can be seen that although the 1,3-propene sultone of the present invention alone shows an excellent effect, when it is used in combination with vinylene carbonate, it is compared with the case where the addition amount to the electrolyte is the same. , the self-discharge ratio can be reduced, and the effect of suppressing load characteristics and impedance and deterioration can be maintained at a high level.
reference example 2
[0133] A laminated battery was produced, and the amount of gas generated in the battery in a high-temperature storage test was measured.
[0134] The laminated battery was fabricated as follows.
[0135] The non-aqueous electrolytic solution prepared in Reference Example 1 above was used as the non-aqueous electrolytic solution.
[0136] The negative electrode is made as follows, 87 parts by weight of natural graphite (LF-18A type manufactured by Zhongyue Black Lead) and 13 parts by weight of the binder polyvinylidene fluoride (PVDF) are mixed, dispersed in the solvent N-methylpyrrolidone, and prepared into natural graphite mortar. Subsequently, this negative electrode paste was coated on a 18 μm thick strip-shaped copper negative electrode collector, and dried. The thickness of the natural graphite electrode is 110 μm. It was die cut to 85mm x 50mm and fitted with copper leads.
[0137] LiCoO 2 Electrodes were fabricated as follows. LiCoO 2 (Honmotosou FMC Enaji-System...
PUM
| Property | Measurement | Unit |
|---|---|---|
| face spacing | aaaaa | aaaaa |
| face spacing | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com