Nano granules of solid lipid of tanshinone and its preparation method
A technology of solid lipid nanometer and tanshinone, which is applied in the directions of liposome delivery, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve problems such as small targeting effect of injections, low bioavailability, and influence on therapeutic effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
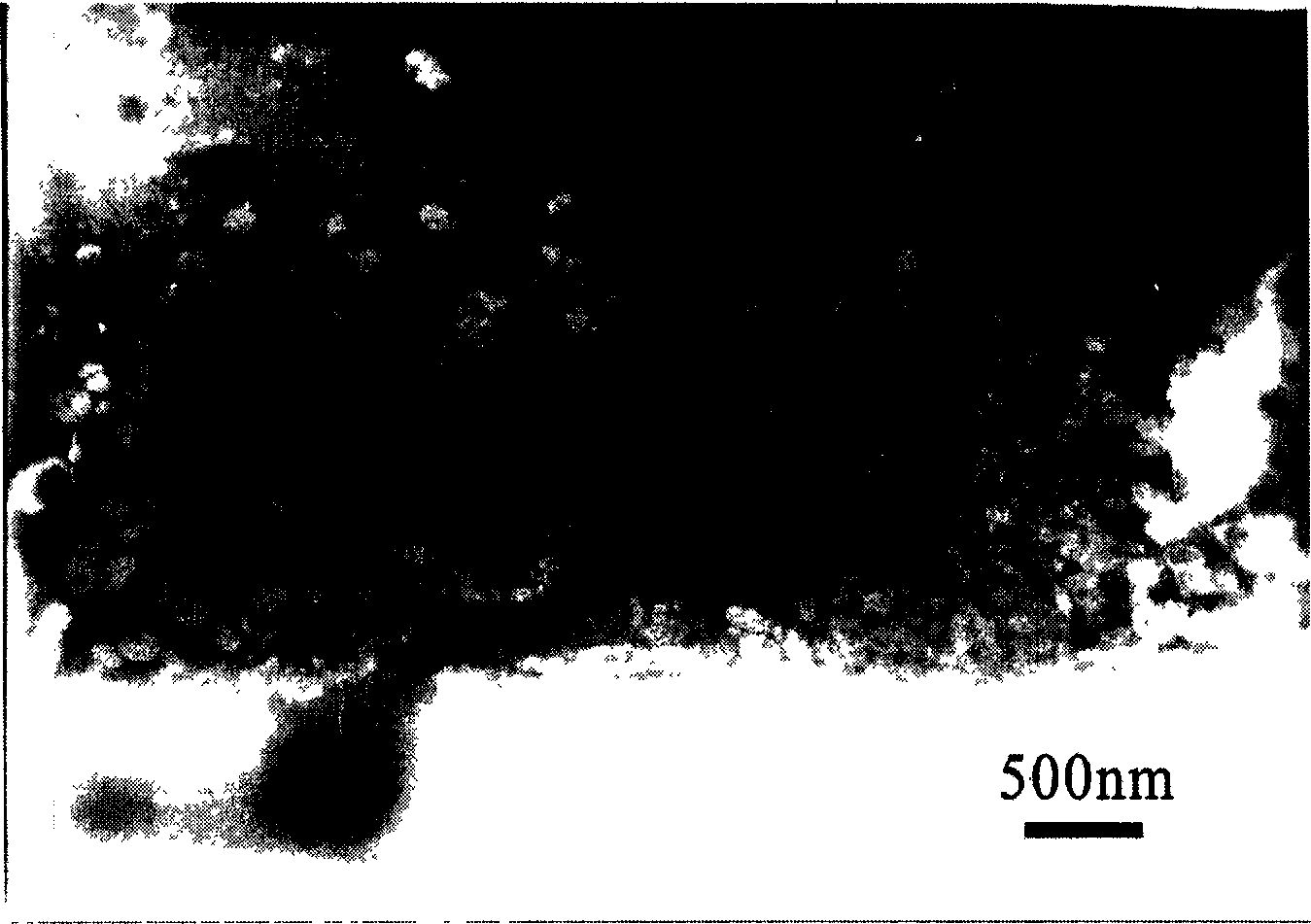
[0054] 10 mg of tanshinone (containing 30% of tanshinone IIA) and 100 mg of stearic acid were dissolved in acetone, and 200 mg of soybean lecithin was dissolved in a small amount of ethanol, and the two parts were mixed to form an organic phase. 350 mg of Poloxamer 188 was dissolved in redistilled water to form the aqueous phase. The organic phase at 75°C was slowly injected into the aqueous phase at the same temperature (75°C) with a syringe, and stirred at constant temperature until a transparent system was formed. The clear system was evaporated under reduced pressure on a rotary evaporator to remove the organic solvent and concentrated to 2 / 3 volume. The concentrated system is quickly mixed in an aqueous phase at 0-2° C., stirred and cooled for 2 hours to form a suspension of solid lipid nanoparticles. Filter through a microporous membrane to remove large particles, and store at 4°C.
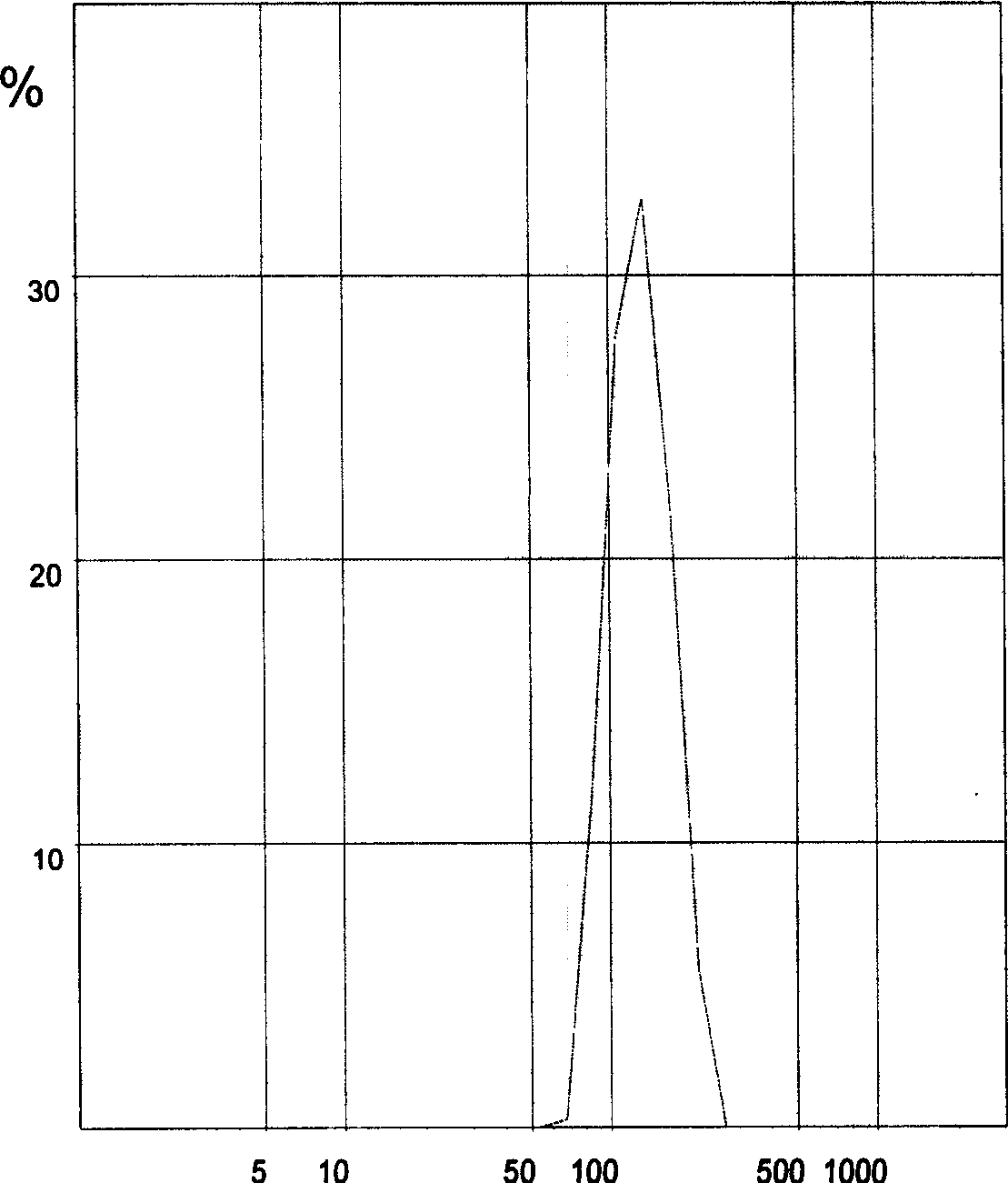
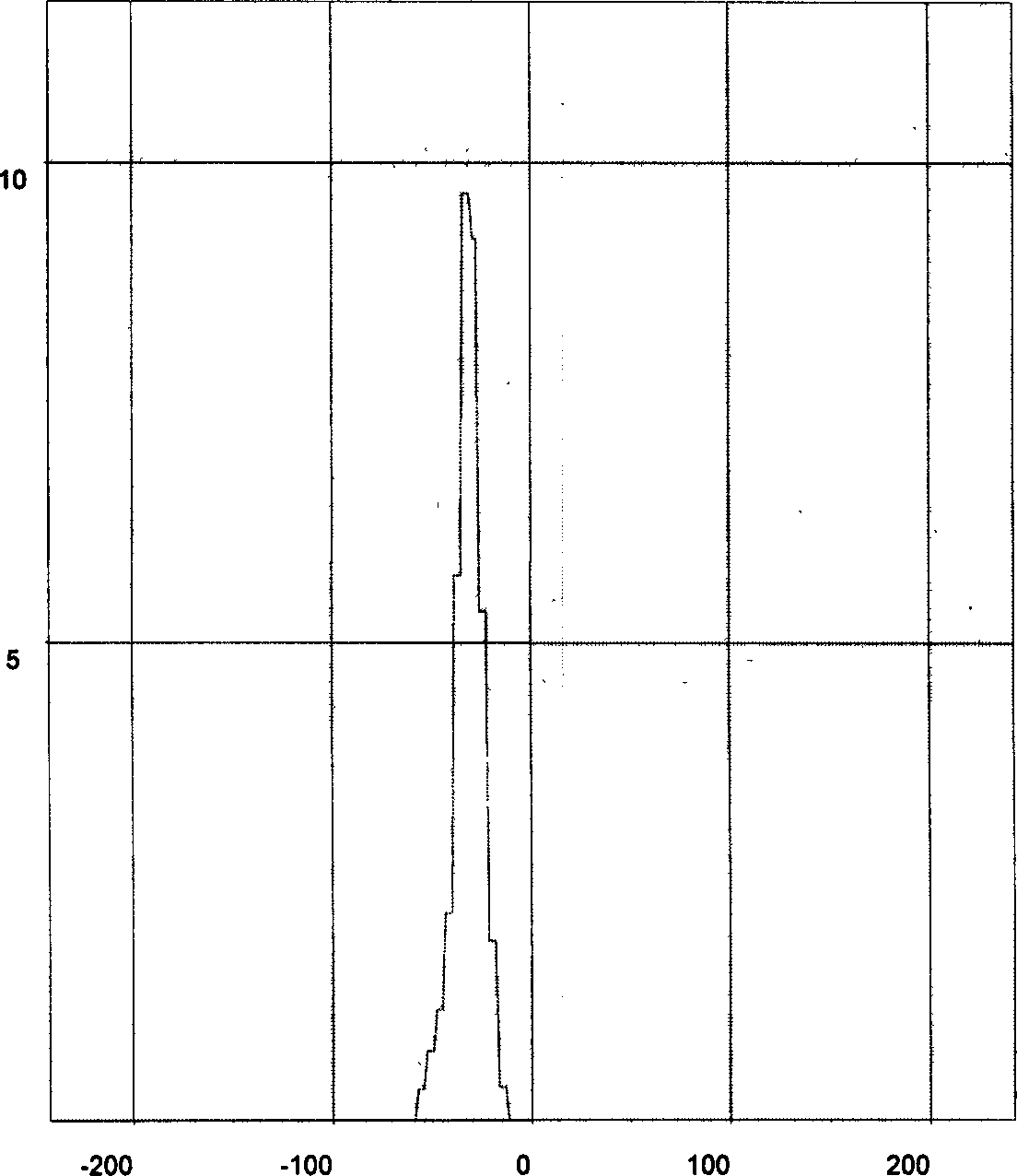
[0055] Detection: The average particle size of tanshinone solid lipid nanoparticles wa...
Embodiment 2
[0057] 10 mg of tanshinone (containing 40% of tanshinone IIA), 150 mg of glycerol monostearate and 300 mg of soybean lecithin were dissolved in ethanol to form an organic phase. 300 mg of Cremophor EL (polyoxyethylene castor oil) was dissolved in redistilled water to form the aqueous phase. The organic phase at 75° C. was slowly injected into the aqueous phase at the same temperature with a syringe, and stirred at constant temperature until a transparent system was formed. The clear system was evaporated under reduced pressure with a rotary evaporator to remove the organic solvent and concentrated to 1 / 4 volume. The concentrated system is quickly mixed in an aqueous phase at 0-2° C., stirred and cooled for 2 hours to form a suspension of solid lipid nanoparticles. Filter through a microporous membrane to remove large particles, and store at 4°C.
[0058] Detection: The average particle size of tanshinone solid lipid nanoparticles was 110.0 nm, and the encapsulation efficienc...
Embodiment 3
[0060] 12 mg of tanshinone (containing 70% of tanshinone IIA), 90 mg of stearic acid and 30 mg of stearyl alcohol were dissolved in isopropanol, and 400 mg of soybean lecithin was dissolved in a small amount of ethanol, and the two parts were mixed to form an organic phase. Dissolve 300 mg of Tween-80 in redistilled water to form the aqueous phase. The organic phase at 75° C. was slowly injected into the aqueous phase at the same temperature with a syringe, and stirred at constant temperature until a transparent system was formed. The clear system was evaporated under reduced pressure on a rotary evaporator to remove the organic solvent and concentrated to 2 / 5 volume. The concentrated system is quickly mixed in an aqueous phase at 0-2° C., stirred and cooled for 2 hours to form a suspension of solid lipid nanoparticles. Filter through a microporous membrane to remove large particles, and store at 4°C.
[0061] Detection: The average particle size of tanshinone solid lipid na...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com