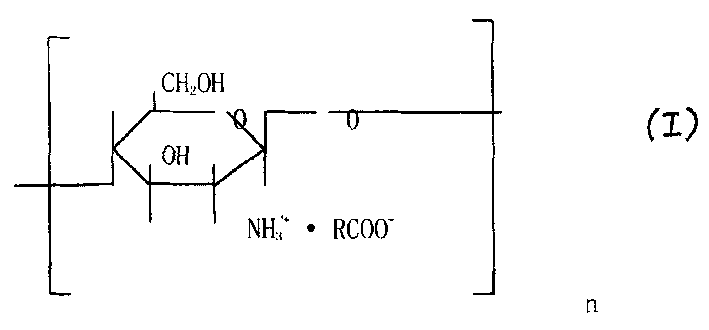
Chitosan derivatives, their preparation and medical composition containing the same as active component
A compound and drug technology, applied in the field of chitosan derivatives, can solve the problems of corneal ulcer and corneal goblet cell differentiation and reduction, and achieve the effects of good chemical property stability, good water solubility, and improved moisturizing performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of Chitosan Lactate
[0054] Chitosan with a viscosity average molecular weight of 50,000-400,000 is 100g, soaked in 1300mL of deionized water for 0.5-1 hour, added 132ml of lactic acid, controlled the reaction temperature at 20°C, and reacted for 4-6 hours. Concentrate by nanofiltration, and the obtained concentrated solution is subjected to vacuum freeze-drying to obtain the final product (purity above 99%, modification rate at 99%, white sheet, easy to dissolve at room temperature).
[0055] NMR analysis results of chitosan lactate
[0056] Hydrogen atom Chemical shift (peak intensity) Number of hydrogen atoms
[0057] H1 4.73725(0.9411) 1
[0058] H2 3.03592(1.000) 1
[0059] H5 3.51608(1.2129) 1
[0060] H3, 4, 6, 6' 3.66585, 3.76325(3.7320) 4
[0061] Ha 1.19985-1.29622(3.5409) 3
[0062] Hb 4.14336-4.16975(1.2697) 1I(Ha) / I(Hb)=3.5409 / 1.2697≈3 conforms to CH in the structural formula 3(a) CH-OH (b) COO - Medium H a / H b Ratio [I(H a ) / 3] / I(...
Embodiment 2
[0097] artificial tears
[0098] Chitosan Lactate 0.5%
[0099] Natural Stabilizer 4%
[0100] NaCl 0.014%
[0101] KCl 0.014%
[0102] CaCl 0.014%
[0103] 1%NaHCOO 3 0.05M
[0104] Preparation method: prepare 1% NaHCO 3 Solution Chitosan lactate, natural stabilizer, NaCl, KCl, CaCl are weighed according to the prescription, dissolved in prepared 1% NaHCO 3 The solution is tested for pH, meets the requirements of artificial tears, and is bottled.
[0105] Artificial tears requirements
[0106] PH: 6-8 (human tears 6.5-7.4)
[0107] Osmotic pressure: 248-370mOsM / kg
[0108] Viscosity: 2.0-6.0mPa.S (human tears 4.0-5.0mPa.S)
[0109] Preservatives: less toxic and side effects (preferably not used)
[0110] Clarity: colorless and transparent
[0111] Safety: The ingredients used are non-toxic, safe and reliable, with good hygroscopicity and moisturizing properties.
Embodiment 3
[0113] Tablets (10,000 pieces):
[0114] Chitosan Lactate 1kg
[0115] Beating starch (10%) 0.5kg
[0116] Starch 200g
[0117] Magnesium Stearate 7.5g
[0118] Preparation method: mix chitosan lactate, beating starch and starch, sieve and dry, add magnesium stearate, and press into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com