Synovial membrane cell protein
A protein and cell technology, applied in the field of new proteins, can solve the problems of not elucidating the biological characteristics of cells and not knowing the relationship between RA factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0302] [Example 1] Preparation of anti-synovial cell anti-serum
[0303] Anti-syviocyte anti-serum was obtained using synoviocytes prepared by the method described below as an immunogen. Synovial tissue extracted from 10 rheumatoid arthritis (RA) patients via synovectomy was washed with phosphate-buffered saline (PBS) under sterile conditions. The washed tissue was cut into squares with a size of about 5 mm, and digested with 0.25% trypsin / PBS at 37° C. for 20 minutes. Excess tissue pieces were removed from the digested synovial tissue, and the resulting cells were suspended in Dulbecco's modified Eagle medium (Virology, 8, 396, 1959) (10% FCS-DMEM) containing 10% fetal calf serum, at 5% CO 2 , 37°C for 24 hours in a sterile cell culture dish. The culture supernatant was discarded, washed with 10% FCS-DMEM, and unattached cells were removed to obtain cells adhered to the plate, which were synoviocytes obtained from rheumatoid patients (The Journal of Clinical Investigation,...
Embodiment 2
[0306] [Example 2] Gene Cloning of Antigen (Synoviolin) Recognized by Anti-Syvioviocyte Antiserum
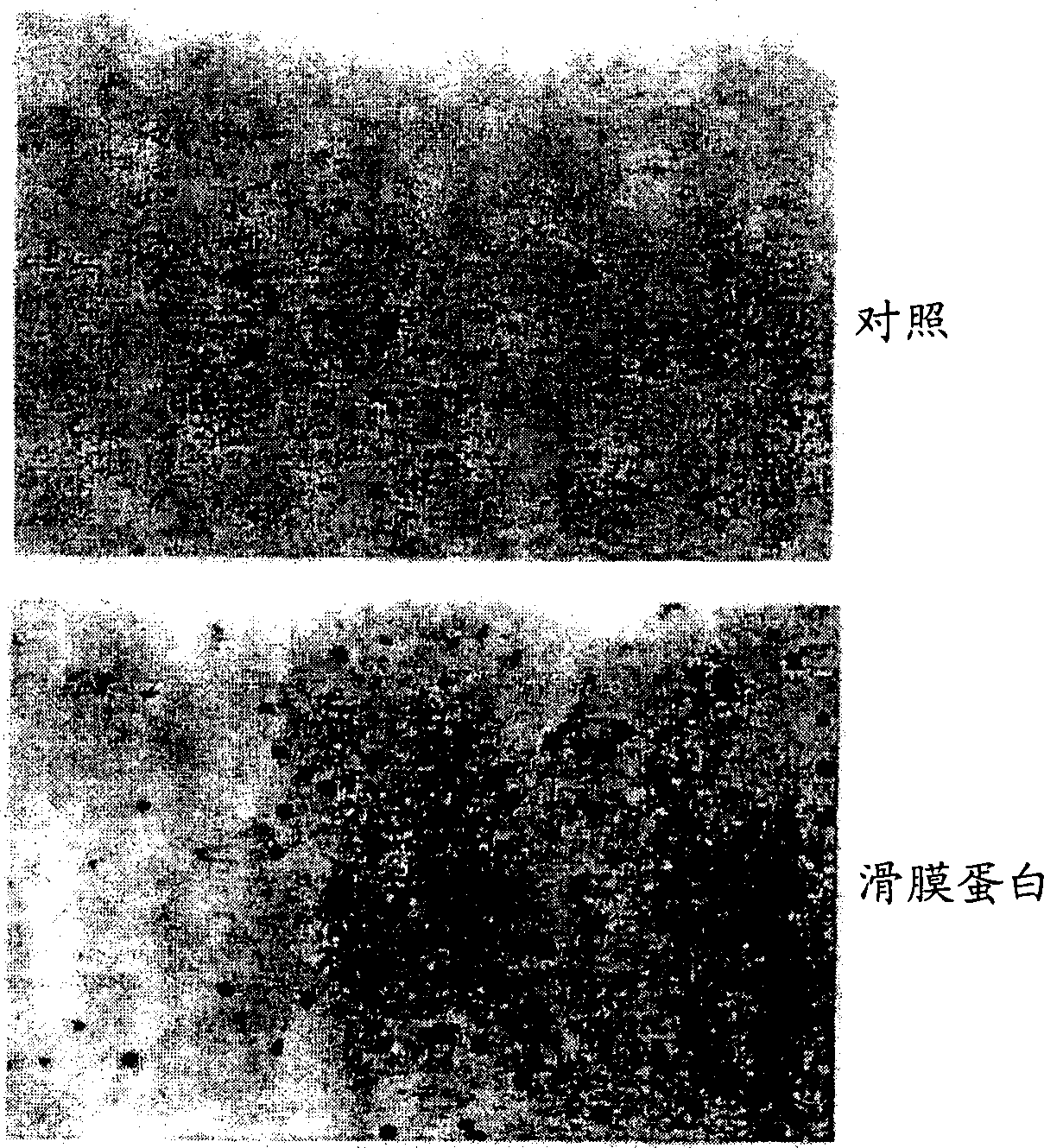
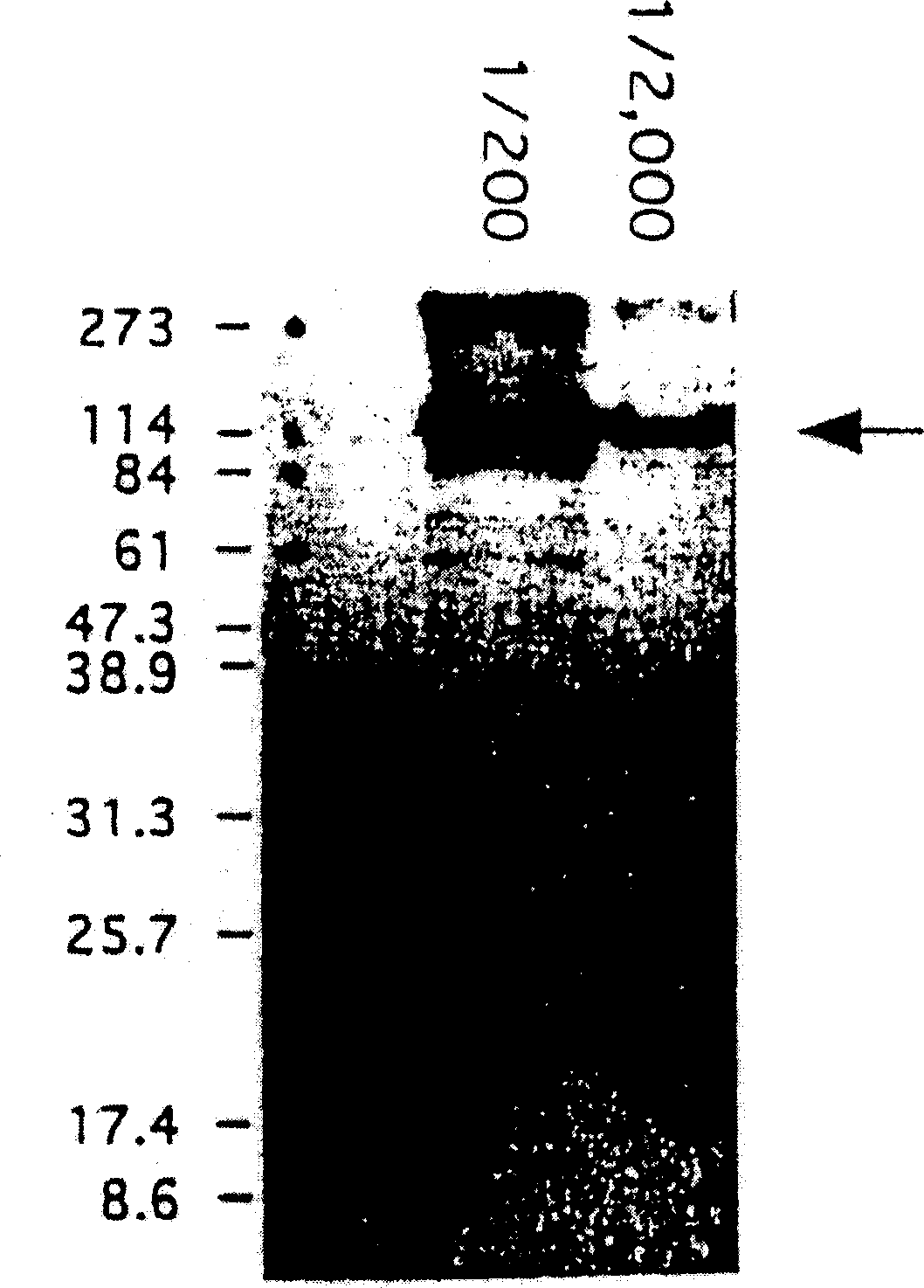
[0307] Total RNA from 10 RA patient synoviocytes obtained in Example 1 was extracted using the acid guanidine / phenol chloroform method, and mRNA was purified using poly T beads (Analytical Biochemistry, 162, 159, 1987). A cDNA library of RA patient synoviocytes was prepared by a conventional method using λZAP vector (Stratagene). Using the picoBlue immune screening kit (Stratagene), carry out immune screening with the anti-synovial cell antiserum of Example 1 above ( figure 1 ). The resulting positive clone (phage) was transformed into plasmid pBluescript II SK(+) with helper phage. Using M13PrimerM4 and M13PrimerRV (Takara), based on the dye terminator method (Proc.Natl.Acad.Sci.USA., 74, 5463, 1977), the ABI PRISM377 DNA sequencer (PERKIN ELMER) was used to measure the number of inserted pBluescript II SK (+) The nucleotide sequence of DNA. Determination of the nucleotide...
Embodiment 3
[0308] [Example 3] Partial synovialin recombinant protein expressed in Escherichia coli
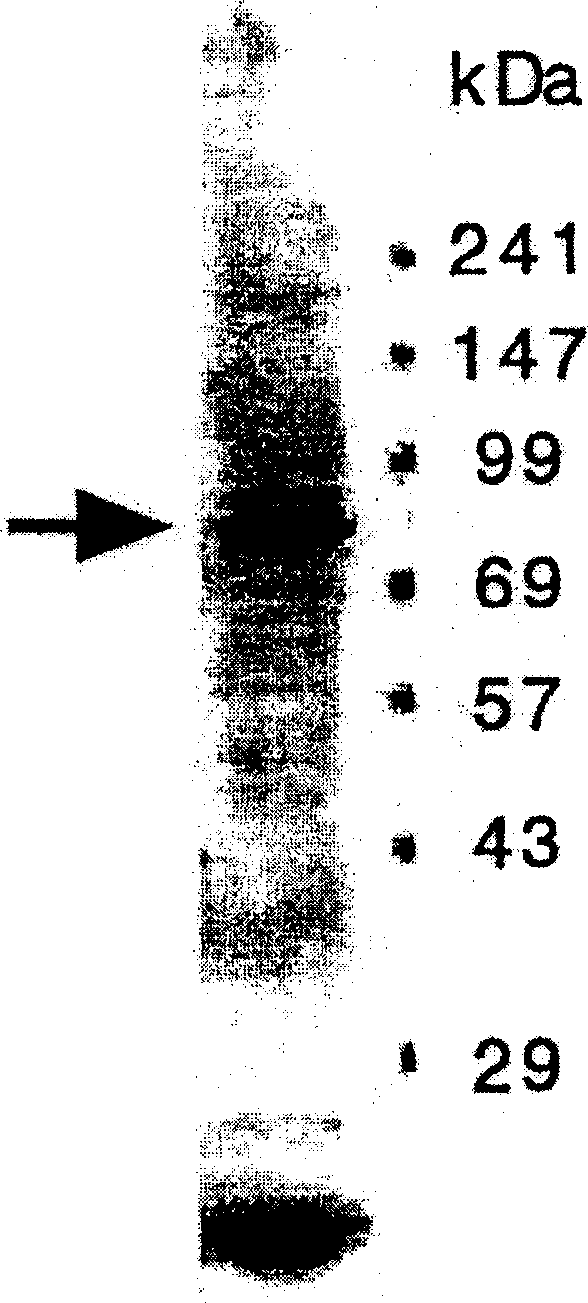
[0309] From the cDNA clones obtained by immunoscreening using anti-synoviocytic antiserum, the cDNA encoding a part of synoviolin (1799 bp; SEQ ID NO: 1, pages 1233-3031) was treated with restriction enzymes EcoRI and XhoI bit), and extract the cDNA. The cDNA with the sequence recognized by EcoRI / XhoI at the end was inserted into glutathione S-transferase (GST) fusion protein expression vector pGEX-5X-3 for subcloning. By heat-shocking at 42°C for 45 seconds, pGEX-5X-3 into which a part of synoviolin cDNA was inserted was introduced into BL21 E. coli strain to obtain BL21 / synoviolin-GST gene / pGEX-5X-3. Cultivate the BL21 strain in LB medium containing 0.1 mg / ml ampicillin, add 0.1 mM isopropylthio-β-D-galactoside (IPTG), and culture at 37°C for another 2 hours to induce the fusion protein expression. After washing BL21 recovered by centrifugation with PBS, BL21 was digested with 1 mg / m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com