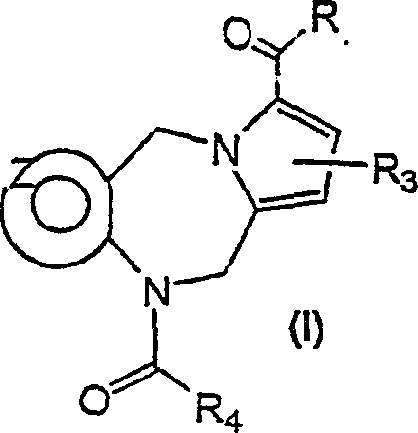
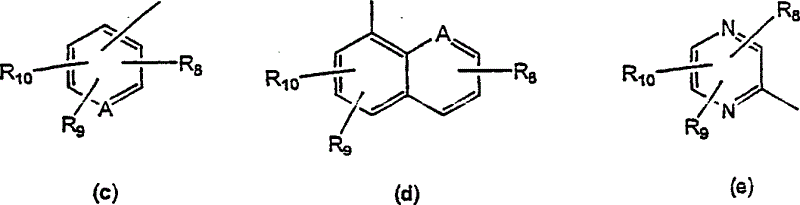
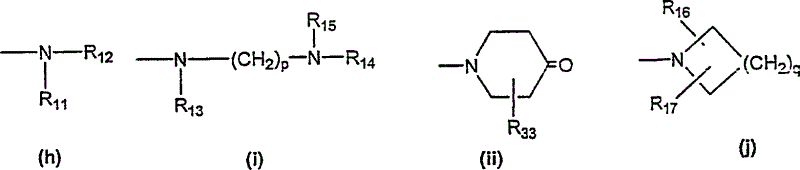Tricyclic diazepines as tocolytic oxytocin receptor antagonists
A technology of halogen and medicinal salt, applied in the field of novel tricyclic diazepines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0273] 10-[(2-methyl-2'-trifluoromethyl-[1,1'-biphenyl]-4-yl)carbonyl]-10,11-dihydro-5H-pyrrolo[2,1- c][1,4]benzodiazepine-3-carboxylic acid
[0274] Step A. Methyl 4-bromo-3-methylbenzoate
[0275] To a suspension of 4-bromo-3-methylbenzoic acid (10.0 g, 46.5 mmol) in methanol (125 mL) was added concentrated sulfuric acid (1 mL). The reaction was heated at reflux overnight and a homogeneous solution was obtained after several minutes of heating. After cooling, methanol was removed under vacuum and the residue was dissolved in dichloromethane and washed with saturated aqueous sodium bicarbonate. The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to afford 10.2 g of the title compound as a brown solid, m.p. 41-43°C.
[0276] 1 H NMR (DMSO-d 6 , 400MHz): δ2.39(s, 3H), 3.85(s, 3H), 7.64-7.72(m, 2H), 7.88-7.89(m, 1H).
[0277] MS [EI, m / z]: 228 [M] + .
[0278] Elemental Analysis: C 9 h 9 BrO 2 Calculated for: C 47.19, H 3.90....
Embodiment 2
[0305] (4-methyl-piperazin-1-yl)-[10-(2-methyl-2'-trifluoromethyl-[1,1'-biphenyl-4-yl)-carbonyl]-10, 11-Dihydro-5H-pyrrolo[2,1-c][1,4]benzodiazepin-3-yl-methanone
[0306] 10-[( 2-Methyl-2'-trifluoromethyl-[1,1'-biphenyl]-4-yl)-carbonyl]-10,11-dihydro-5H-pyrrolo[2,1-c][ 1,4] A suspension of benzodiazepine-3-carboxylic acid (1.65 g, 3.36 mmol). After gas evolution had ceased, the reaction mixture was refluxed for another 15 min. The cooled solution was evaporated to dryness to give the crude acid chloride as a brown solid. The acid chloride was then dissolved in dichloromethane (10 mL) and slowly added to 1-methylpiperazine (1.5 mL, 13.5 mmol) and N,N-diisopropylethylamine (3.5 mL, 20.1 mmol) in dichloro methane (25 mL). After stirring overnight, water was added to quench the reaction. The organic layer was washed sequentially with 1N hydrochloric acid, 1N sodium hydroxide and brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to give a brown o...
Embodiment 3
[0311] 10-[(2-methyl-2'-trifluoromethyl-[1,1'-biphenyl]-4-yl)-carbonyl]-10,11-dihydro-5H-pyrrolo[2,1 -c][1,4]benzodiazepine-3-carboxylic acid bis-(3-dimethylamino-propyl)amide
[0312] 10-[ (2-Methyl-2'-trifluoromethyl-[1,1'-biphenyl]-4-yl)-carbonyl]-10,11-dihydro-5H-pyrrolo[2,1-c] A suspension of [1,4]benzodiazepine-3-carboxylic acid (0.50 g, 1.02 mmol). After gas evolution had ceased, the reaction mixture was refluxed for an additional 15 minutes. The cooled solution was evaporated to dryness to give the crude acid chloride as a brown solid. The acid chloride was then dissolved in dichloromethane (5 mL) and slowly added to bis-(3-dimethylamino-propyl)-amine (0.90 mL, 4.04 mmol) and N,N-diisopropylethylamine ( 1.1 mL, 6.31 mmol) in dichloromethane (5 mL). After stirring for 2 hours, water was added to quench the reaction. The organic layer was washed sequentially with 1N hydrochloric acid, 1N sodium hydroxide and brine, dried over anhydrous sodium sulfate, filtered and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com