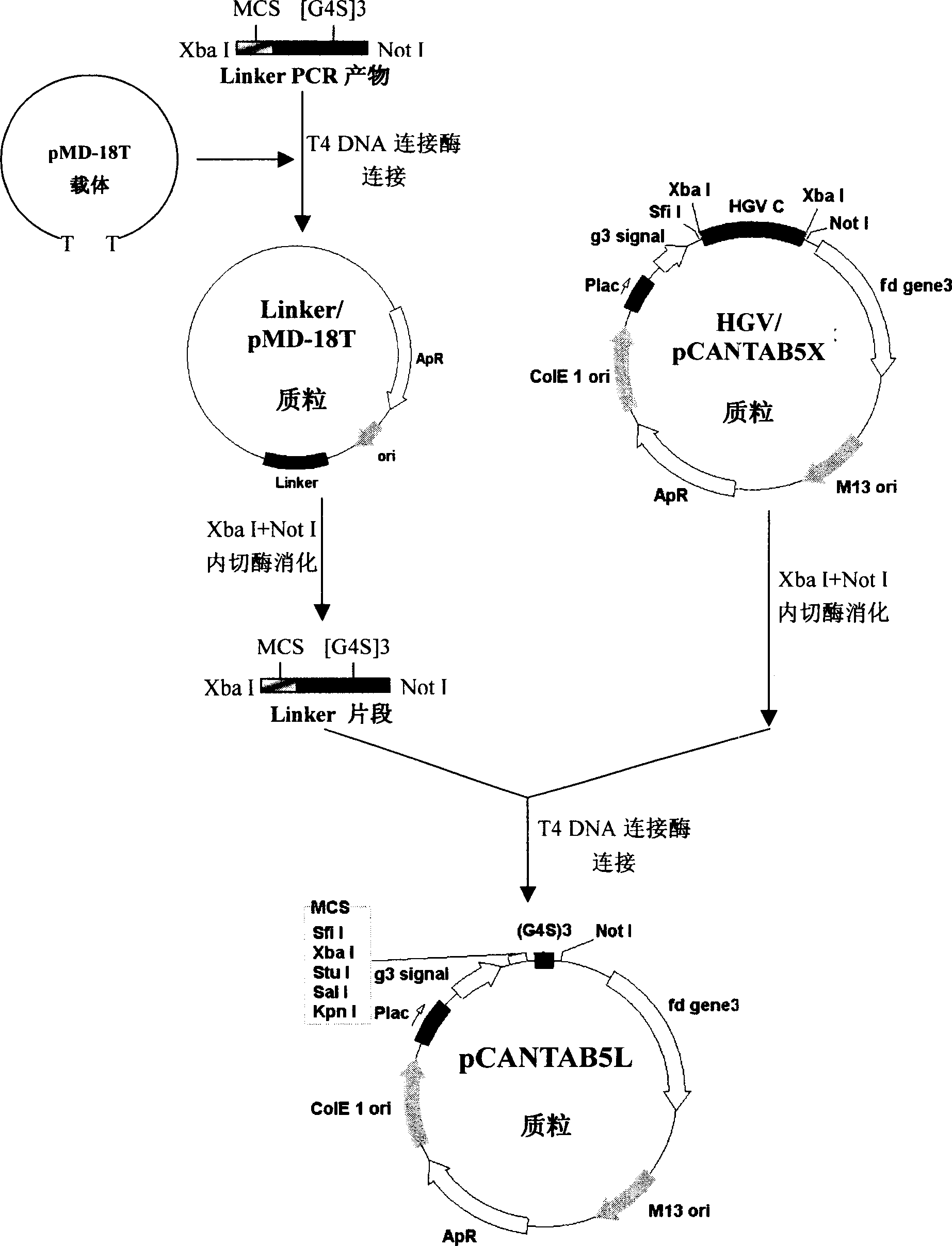
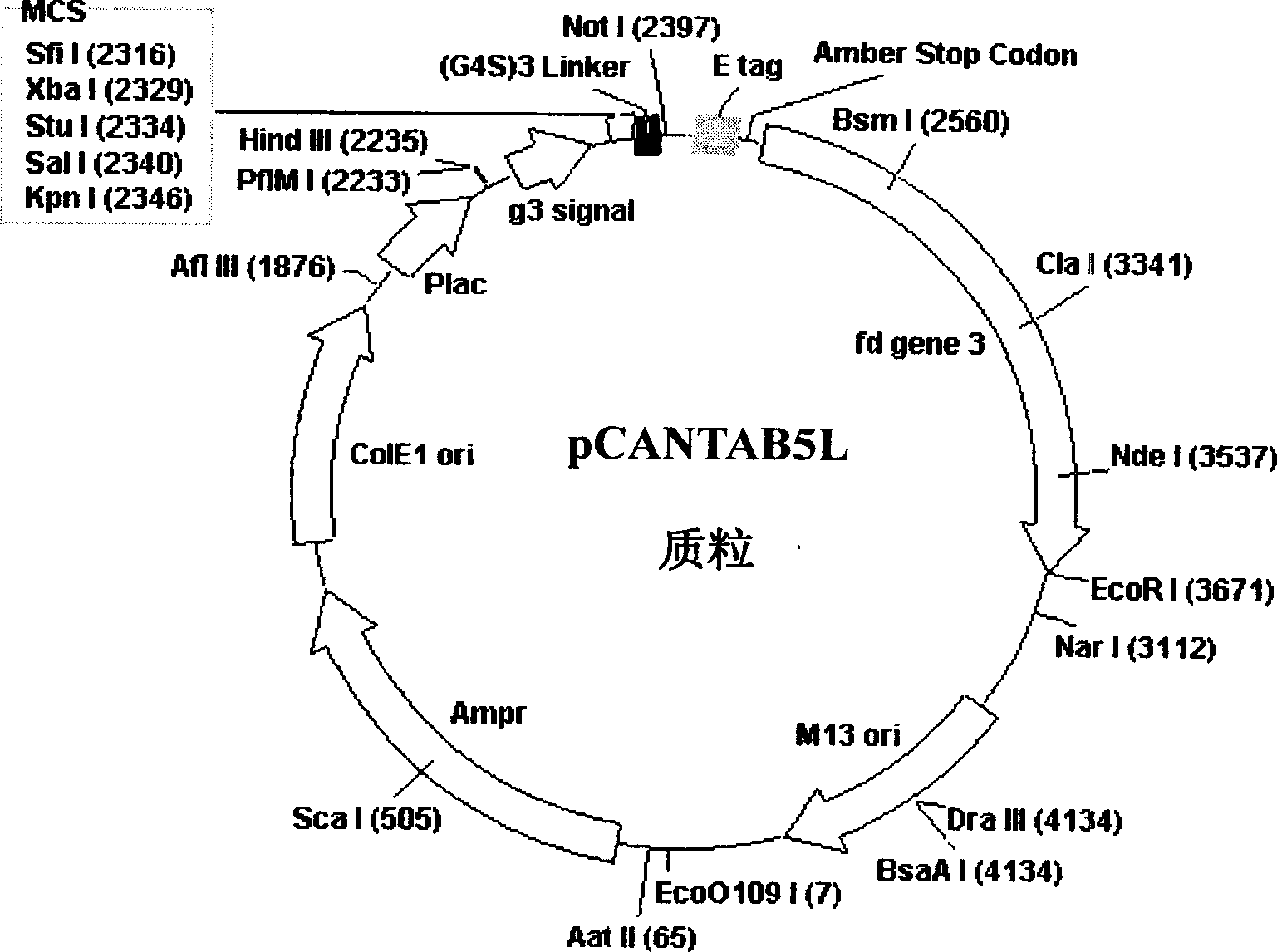
Phasmid display carrier pCANTAB5L
A phagemid and carrier technology, applied in the field of bioengineering, can solve the problems of reduced yield of recombinant phage, less number of endonuclease sites, inconvenient genetic engineering operation, etc., to expand the capacity of the phage display peptide library, which is beneficial to the activity The effect of maintaining and improving cloning efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Application of recombinant phagemid vector pCANTAB5L to display recombinant human lymphotoxin (rhLT) random variant library
[0049] In order to confirm whether the recombinant phagemid vector pCANTAB5L can correctly display the functional protein variant library, the recombinant human lymphotoxin (rhLT) random variant library cloned on the pGEM-T easy vector was digested with Xba I and Kpn I double enzymes and then directed Cloned in the multiple cloning site of pCANTAB5L, a recombinant phage library displaying random variants of rhLT was constructed. The specific operation process is as follows:
[0050] (1) Construction of recombinant phage displaying rhLT random variant library
[0051] The rhLT / pGEM-T easy plasmid contains the recombinant human lymphotoxin derivative rhLT random variant library gene, and the library capacity is 1×10 6The recombinant human lymphotoxin expressed by the rhLT gene is missing 23 amino acids at the N-terminal, and contains a...
Embodiment 2
[0057] Example 2: Application of recombinant phagemid vector pCANTAB5L to display macromolecular polypeptide-staphylococcal protein A derivative (Mu-protein A):
[0058] In order to test whether pCANTAB5L can correctly display macromolecular polypeptides, the gene of Staphylococcus A protein derivative (Mu-protein A) was digested with Xba I and Kpn I double enzymes from the pGEM-T easy vector and cloned into the multiple cloning site of pCANTAB5L In the middle point, a recombinant phage displaying Mu-protein A was constructed. The specific operation process is as follows:
[0059] (1) Construction of recombinant phage displaying Mu-protein A:
[0060] Staphylococcus protein A (Mu-protein A) consists of 360 amino acids and contains 5 antibody binding domains. The Mu-protein A gene is cloned on the Mu-protein A / pGEM-T easy plasmid, and its 5' end contains an Xba I restriction site, and its 3' end contains a Kpn I restriction site. Mu-protein A / pGEM-T easy plasmid was recovere...
Embodiment 3
[0081] Embodiment 3: Application of recombinant phagemid vector pCANTAB5L to display human interferon (hIFNαA-2b):
[0082] In order to compare whether the recombinant phagemid vector pCANTAB5L containing the [G4S]3 polypeptide linker can maintain its biological activity better than the original pCANTAB5X phagemid when displaying functional proteins, human interferon αA-2b (hIFNαA-2b) Clone into the multiple cloning site of pCANTAB5L, construct the recombinant phage displaying hIFNαA-2b, and use the recombinant phage displaying hIFNαA-2b using the pCANTAB5X vector [Wu Xiaolan et al., Journal of Second Military Medical University, 2000, 23(4): 403] Interferon activity comparison. The specific operation process is as follows:
[0083] (1) Construction of recombinant phage displaying hIFNαA-2b:
[0084] The hIFNαA-2b / pGEM-T easy plasmid contains the human interferon αA-2b gene, which encodes an interferon consisting of 166 amino acids. The 5' end of the hIFNαA-2b gene contains...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com