Preparation method of multi-copy golden pomfret flavor peptide, expression vector and recombinant bacteria
An umami peptide and multi-copy technology, applied in the field of food additives, can solve the problems of difficult recovery, difficult expression, and cumbersome overall process, and achieve the effects of high cloning efficiency and fast and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
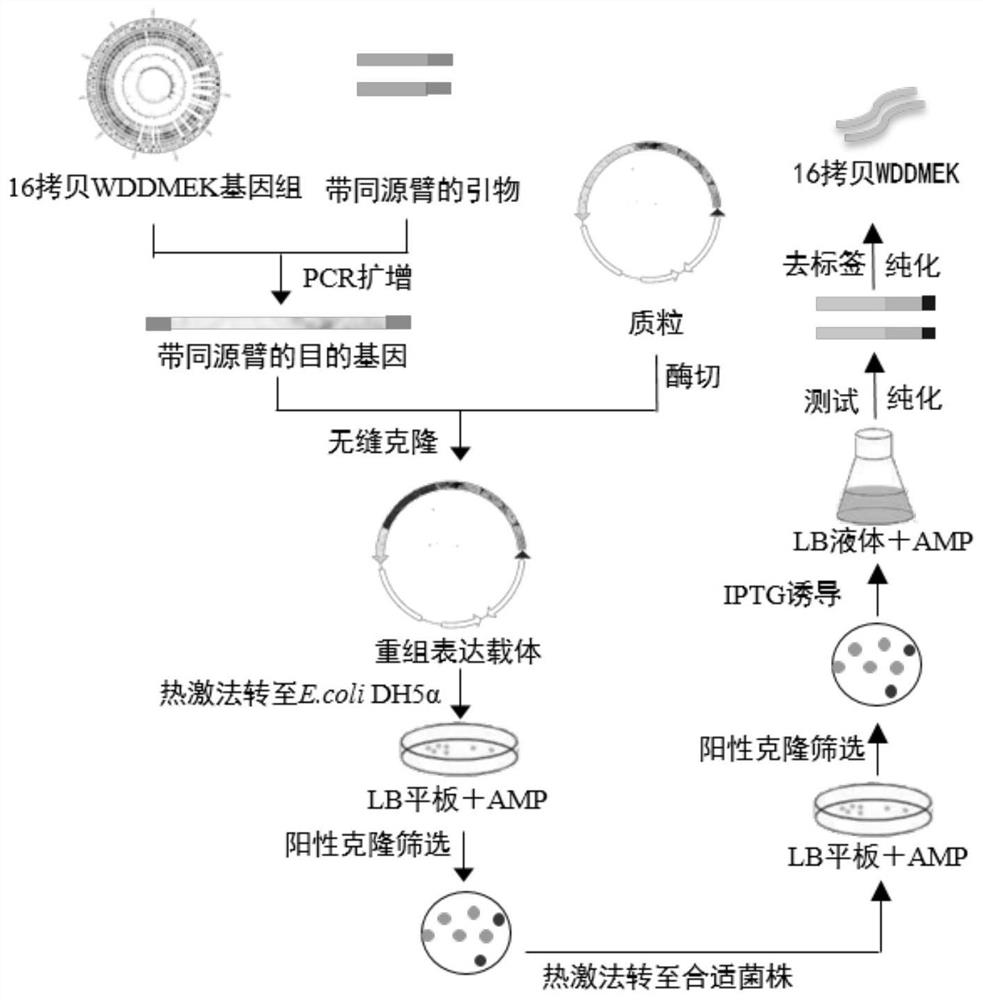
[0032]Example 1: Construction example of the vector
[0033]1.1 Purpose gene sequence amplification
[0034]The gene sequence of the fresh-flavored peptide graphic is obtained by the NCBI database, according to the sequence of the carrier and the target gene, the enzyme dug point and primer are designed with Primer Premier 5.0 software and synthesized, and the plasmid obtained by the obtained cDNA or the plain segment is a template. Gene's cloning.
[0035]Gene sequence SEQ ID NO: 2:
[0036]atgggtcaccatcaccatcaccatatgtcggactcagaagtcaatcaagaagctaagccagaggtcaagccagaagtcaagcctgagactcacatcaatttaaaggtgtccgatggatcttcagagatcttcttcaagatcaaaaagaccactcctttaagaaggctgatggaagcgttcgctaaaagacagggtaaggaaatggactccttaagattcttgtacgacggtattagaattcaagctgatcagacccctgaagatttggacatggaggataacgatattattgaggctcacagagaacagattggtggcTGGGACGATATGGAAAAATGGGATGACATGGAAAAGTGGGACGACATGGAAAAATGGGACGATATGGAAAAGTGGGATGATATGGAAAAATGGGACGATATGGAAAAATGGGACGACATGGAAAAATGGGATGATATGGAAAAATGGGATGACATGGAAAAATGGGACGACATGGAAAAGTGGGATGACATGGA...
Embodiment 2
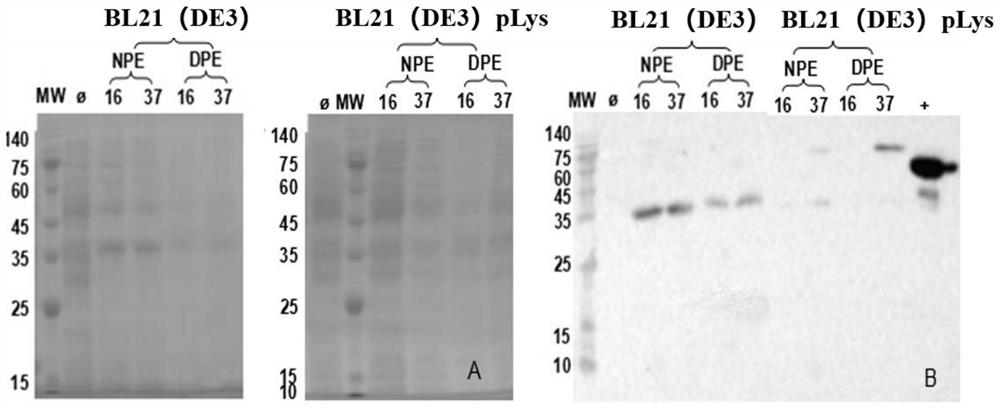
[0061]Example 2: Activation and induction of strains, expression test
[0062]1. Pick the single colonies containing recombinant plasmids from the plate, inoculated in 5 mLlb medium containing cardamycin antibiotic (50 ug / ml), 37 ° C overnight culture.
[0063]2. Take 200 μL in a 20 ml LB medium containing resistance, 37 ° C culture to OD600 = 0.6.
[0064]3. Expression test of two strains: BL21 (DE3), BL21 (DE3) PLYS. A 1 ml of un-induced bacteria was placed in a sterilized 2 ml of centrifuge tube as an undressed control. Direct OD600 to 0.5 ~ 0.6 were cultured in the remaining bacteria to add IPTG to a final concentration of 1 mm, and the bacterial liquid obtained after IPTG and the undened control were cultured in the following, respectively: 16 ° C overnight culture, 37 ° C culture 4h .
[0065]4. Samples of different expressions were collected, and 12,000 rpm was centrifuged for 1min centrifugation. With a 200 ul of PBS suspension, ultrasonic crushing cells, ultrasonic time 2s, interval ...
Embodiment 3
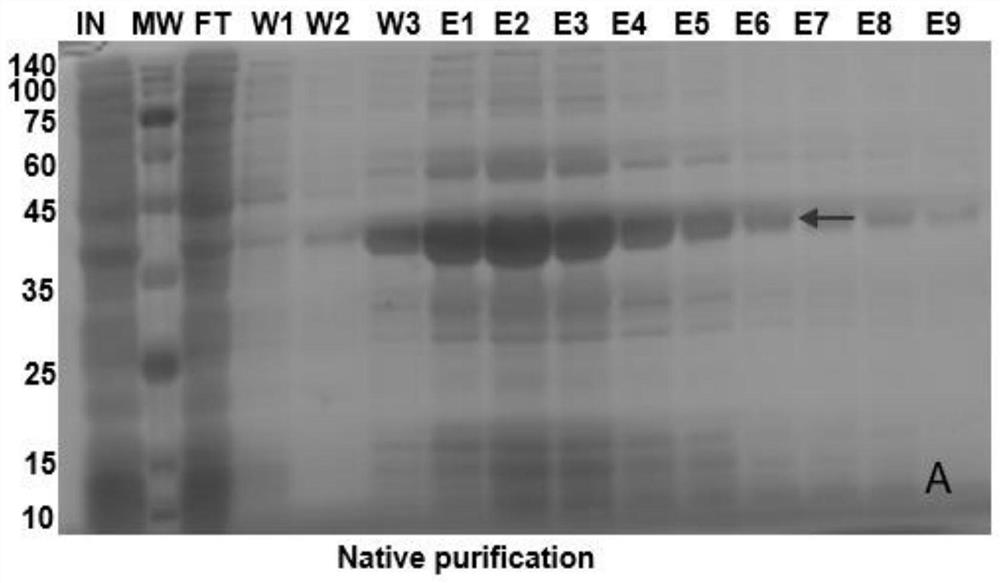
[0068]Example 3: 1000ml amplification and purification
[0069]1. Sample treatment: Select the best induction expression conditions: 16 WDMEK protein with expression strain BL21, 1 mM IPTG, 37 ° C treatment for 4 h. When culturing 1000 ml of bacteria, when the OD600 was detected for a certain period of time, IPTG was added to 1 mM to induce expression, 16 WDMEK protein was induced by 4 h, centrifugal collected induced fungus (when collecting the bacteria), add 10 mlpbs Buffer, ultrasonic treatment, ultrasonic time 2S, interval 2s, total 30min. Collect the supernatant after centrifugation.
[0070]2. Prepare the column
[0071]The resin is placed in a suitable column, and the upper charge is above the resin.
[0072]3. Cleaning the resin: Select 10 times resin volume binding buffer to clean according to the amount of resin.
[0073]Binding Buffer: PBS pH 7.5, 10% Glycerol
[0074]4. Same: The supernatant column will be collected, and this process cannot accelerate. If you need to accelerate, you must ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com