Chargeable lithium ion cell polymer electrolyte and preparing method thereof
A technology of lithium-ion batteries and polymers, applied in secondary batteries, circuits, electrical components, etc., can solve the problems of unstable liquid encapsulation and poor mechanical properties, and achieve high efficiency and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0043] Example 1: Preparation of polymethoxysiloxane (PMOS)
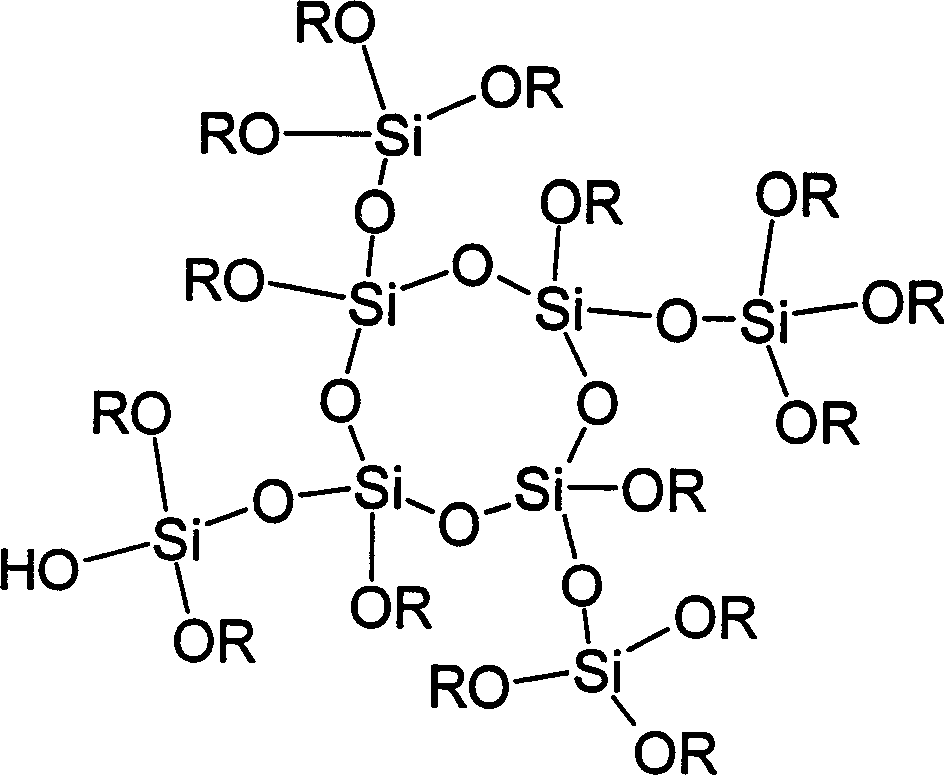
[0044] Put 152g TMOS (1mol) and 128g methanol into a four-necked reaction flask equipped with a thermometer, a mechanical stirrer, and a reflux tube, and stir and mix evenly. Water 18g, HCl 2×10 -4 mol of hydrochloric acid into the dropping funnel. Under vigorous stirring, hydrochloric acid was slowly dropped into the reaction flask for about 1 hour. After the dropwise addition was completed, the temperature was raised to reflux temperature. After reflux for 2 hours, methanol was distilled to 130°C. The temperature was then raised to 150°C and maintained for 3 hours with a stream of dry nitrogen. After cooling to room temperature, 104.0 g of a colorless and clear liquid product PMOS was obtained, with a reaction yield of 97.2%. After characterization, the molecular weight of the product is 1027, and the molecular structure can be expressed as [SiO 0.9769 (OH) 0.0585 (OCH 3 ) 1.991 ] 9.650 .
[0045] In th...
example 2
[0055] Example 2: Preparation of polyacrylmethylsiloxane (PAMOS)
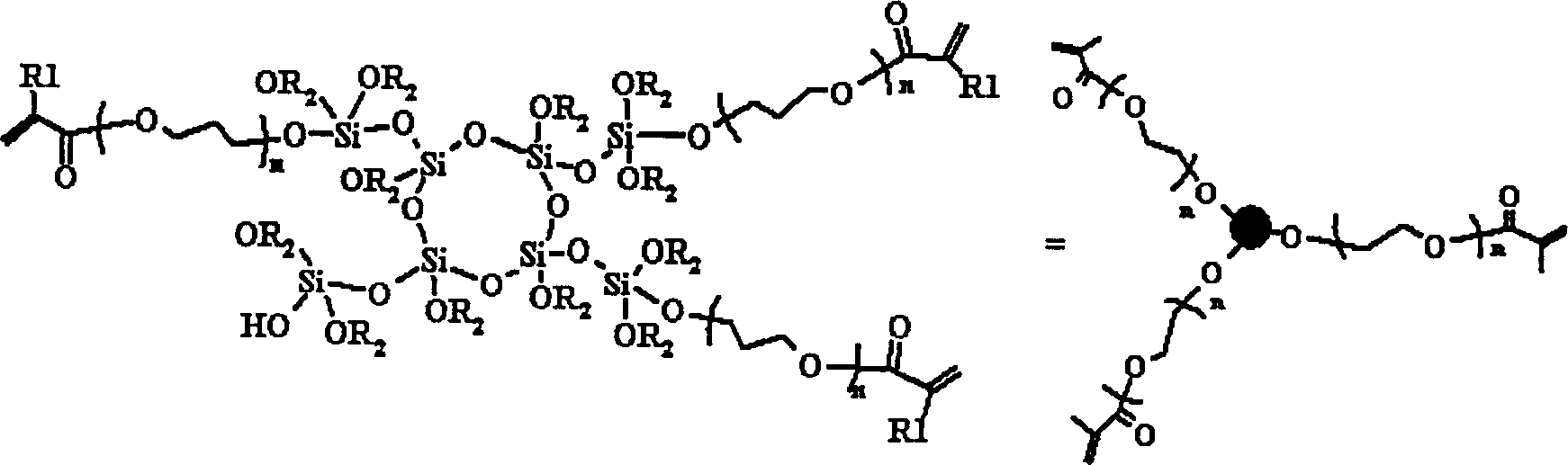
[0056] Add 9.28g (0.08mol) of HEA, 200ppm of dibutyltin dilaurate and 200ppm of hydroquinone monomethyl ether into 10g of PMOS (S-3), heat to 90°C and react for 12h, then remove unreacted HEA under reduced pressure , cooled to obtain 15.02 g of colorless and clear product PAMOS, and the conversion rate of HEA was 74.84%. After characterization, the molecular weight of the product is 1543, and the molecular structure can be expressed as [SiO 0.907 (OH) 0.153 (OCH 3 ) 1.385 (OCH 2 CH 2 OCOCH=CH 2 ) 0.648 ] 9.486 .
[0057] When 10 g of PMOS (S-3) of the same weight was added with different weights of HEA, the molecular weight and structure of the resulting product PAMOS were different, as shown in Table 2.
[0058] The molecular weight and molecular formula of the product PAMOS obtained by different reaction ratios of table 2
[0059] PAMO HEA / S-3 Molecular Weight Molecular Formula
[0060] S (weight ...
example 3
[0072] Example 3 Preparation of Gel State Inorganic-Organic Hybrid Polymer Electrolyte
[0073] SE-2 is mixed evenly with 5wt.% photoinitiator Iragacure 184, and then mixed with different amounts of lithium-ion battery liquid electrolyte (1MLiPF 6 , EC:DEC:DMC=1:1:1w / w) mixed evenly, dripped into the stainless steel button battery case, and exposed to ultraviolet light for 0.5-3 minutes. The gel polymer electrolyte prepared in the coin cell case was covered with a stainless steel cap, packaged, and then tested for electrical conductivity. The results are shown in Table 4.
[0074] Table 4 Composition and Room Temperature Conductivity of Gel Polymer Electrolyte
[0075] No. Polymer Content Liquid Electrolyte Content Room Temperature Conductivity
[0076] 1M LiPF 6 , EC:DEC:DMC=1:1:1 (Scm -1 )
[0077] w / w
[0078] 1 # 5wt.% 95wt.% 8.42×10 -3
[0079] 2 # 10wt.% 90wt.% 3.59×10 -3
[0080] 3 #...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com