Terbium compound electroluminous material and device
A technology of complexes and ligands, applied in the direction of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems that are not comparable to others, and achieve the effect of good electron transport performance and excellent electroluminescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
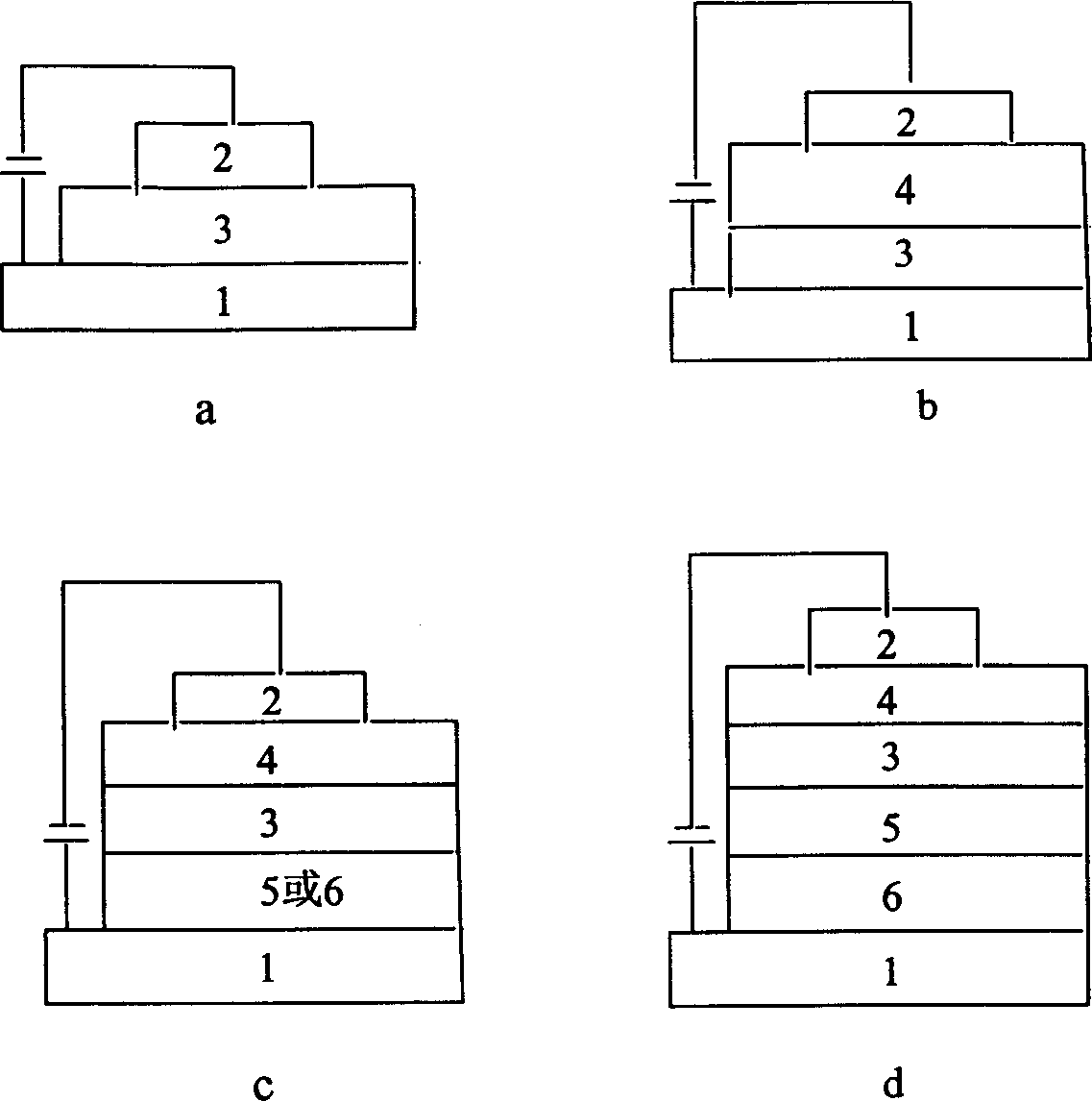
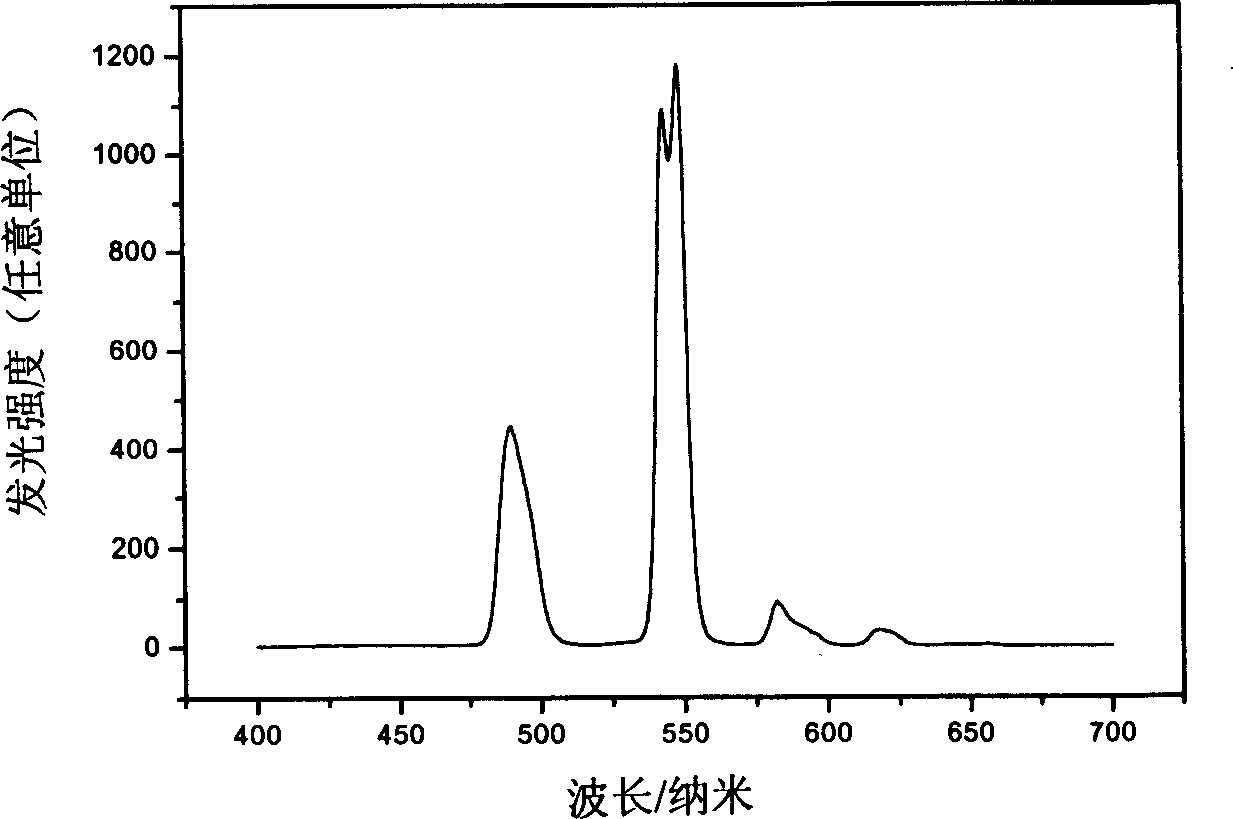
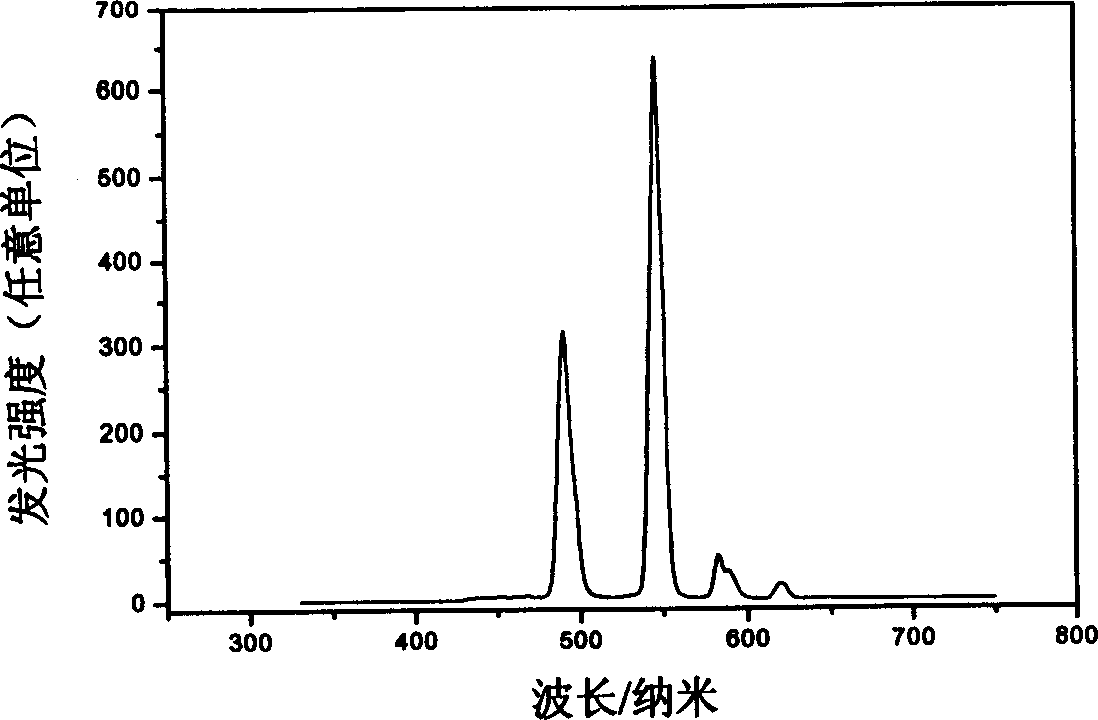
Image
Examples
Embodiment 1
[0078] Example 1: Synthesis of ligand 3-methyl-1-phenyl-4-(2-ethylbutyryl)pyrazolin-5-one (eb-PMP)
[0079] In a dry three-necked flask, add 7.5 g (0.05 mol) of 3-methyl-1-phenylpyrazolin-5-one and 100 ml of anhydrous 1,4-dioxane, heat and stir until dissolved, add 9 The powder of gram (0.18 mol) calcium hydroxide and 1 gram (0.03 mol) barium hydroxide was cooled to room temperature, 8 ml of 2-ethylbutyryl chloride (0.058 mol) was added dropwise, and the reaction was stirred and refluxed for 18 hours. The reaction mixture was poured into 350 ml of deionized water containing 15 ml of concentrated hydrochloric acid, and a red oil was precipitated, extracted with chloroform, washed with deionized water until nearly neutral, dried over anhydrous sodium sulfate for 24 hours, and the obtained liquid was rotary evaporated to dryness, Using petroleum ether:ethyl acetate (20:1) as the eluent, it was separated and purified by silica gel column to obtain 9.6 g of pink oily product with a y...
Embodiment 2
[0081] Example 2: Synthesis of ligand 3-methyl-1-phenyl-4-(2-ethylhexanoyl)pyrazolin-5-one (eh-PMP)
[0082] The synthesis method is the same as in Example 1, but 2-ethylhexanoyl chloride is used instead of 2-ethylbutyryl chloride.
[0083] 1 H-NMR (400 Hz, CDCl 3 )δ: 7.87(2H); 7.45(2H); 7.28(1H); 2.90(1H); 2.48(3H); 1.63(4H); 1.50(4H); 0.94(6H).
Embodiment 3
[0084] Example 3: Synthesis of ligand 3-methyl-1-phenyl-4-(2-tert-butylacetyl)pyrazolin-5-one (te-PMP)
[0085] Following the same procedure as Example 1, but substituting 3,3-dimethylbutyryl chloride for 2-ethylbutyryl chloride, to prepare 3-methyl-1-phenyl-4-(tert-butylacetyl)pyridine oxazolin-5-one (te-PMP). The crude product of this product can be purified by recrystallization from ethanol. Melting point 89-90 ℃.
[0086] 1 H-NMR (400 Hz, CDCl 3 )δ: 7.87(2H); 7.45(2H); 7.28(1H); 2.62(2H); 2.48(3H); 1.86(4H); 1.12(9H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com