Method of catalyzed oxidation of olefin to produce enol, ketenes and epoxy compound
A technology for catalytic oxidation of epoxy compounds, applied in the direction of hydrocarbon oxidation to prepare oxygenated compounds, oxidation to prepare carbonyl compounds, chemical instruments and methods, etc., can solve the problems of losing catalytic effect and not yet put into industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
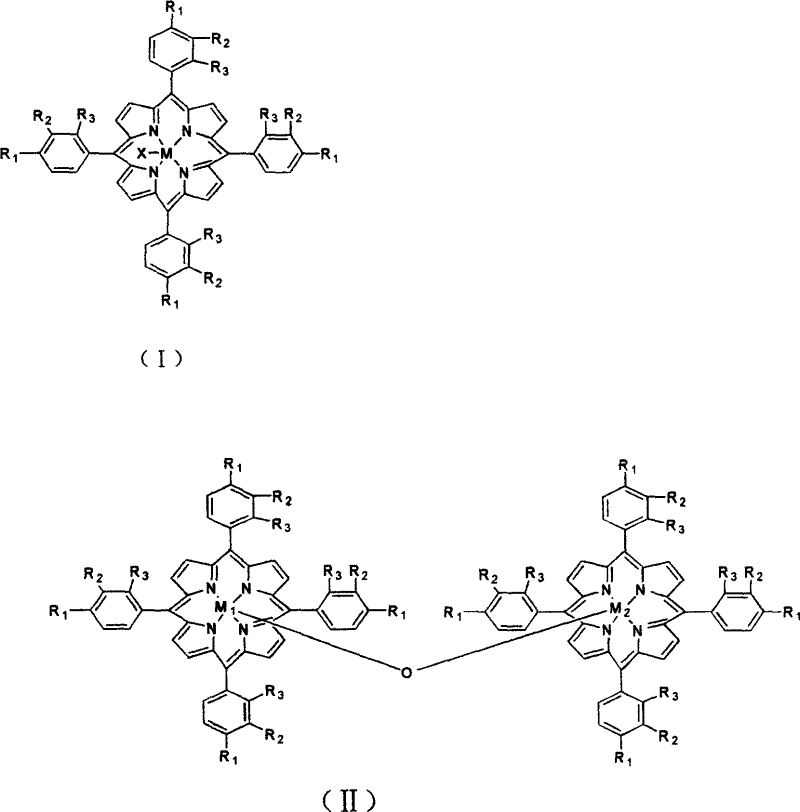
[0015] 5 mg of metalloporphyrins with structural formula (I), R 1 = R 2 = R 3 =CH 3 , M=Mn, and 20mgCu 2 Cl 2 Add 300ml of cyclohexene, and introduce 4atm air. The reactant was stirred at 55° C. for 3 hours, the conversion rate of cyclohexene was 4.2%, and the yield of α, β-cyclohexenol, α, β-cyclohexenone and epoxycyclohexane in the reaction product was 90%.
Embodiment 2
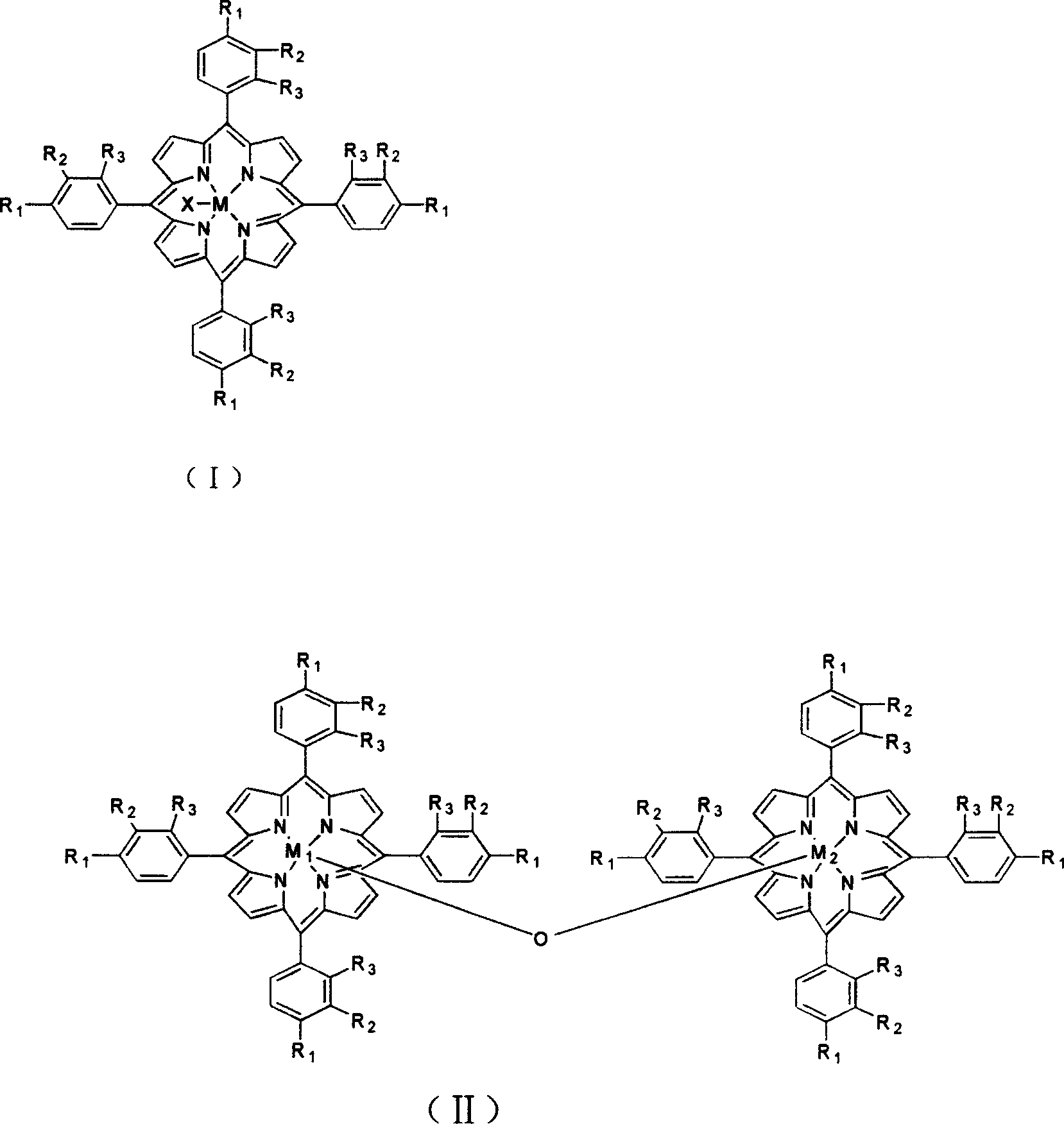
[0017] 3 mg of a metalloporphyrin of formula (II), R 1 = R 2 =OH, R 3 =Cl,M 1 = M 2 =Fe, and 20 mg of sodium phosphate were added in 400 ml of styrene, 5 atm air was introduced, and the reactant was stirred at 80° C. for 4 hours, the conversion of styrene was 10.8%, and the yield of styrene oxide in the reaction product was 60%.
Embodiment 3
[0019] 7 mg of metalloporphyrins of formula (I), R 1 =OCH 3 , R 2 = R 3 = H, M 1 =Co, and 24mg of triethylamine are added in 400ml of 2-butene, feed 8atm air, stir reactant 2 hours at 80 ℃, butene conversion rate is 30.8%, butene oxide and crotonaldehyde are collected in reaction product Rate 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com