Cinnarizone self micro emulsified soft capsule and its preparation method
A self-micro-emulsifying, cinnarizine technology, applied in the field of medicine, can solve the problems of reduced bioavailability and reduced drug dissolution, and achieve the effects of reducing individual differences, improving curative effect, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The composition of the prescription is as follows: (g)
[0034] Cinnarizine 25
[0035] Dimethylisosorbide 100
[0036] Oleic acid 50
[0037] Tween 80 185
[0038] Vitamin E 5
[0039] Made 1000 softgels in total
[0040] Preparation process: Cinnarizine self-microemulsified capsules Preparation method: Weigh an appropriate amount of cinnarizine, add the prescribed amount of dimethyl isosorbide, Tween 80, and oleic acid, and place it in a constant temperature shaking bath at 40C and 100 rpm Shake for about 1.5 hours to make it dissolve, then add antioxidant and stir evenly, and press it into capsules after the liquid drops to room temperature.
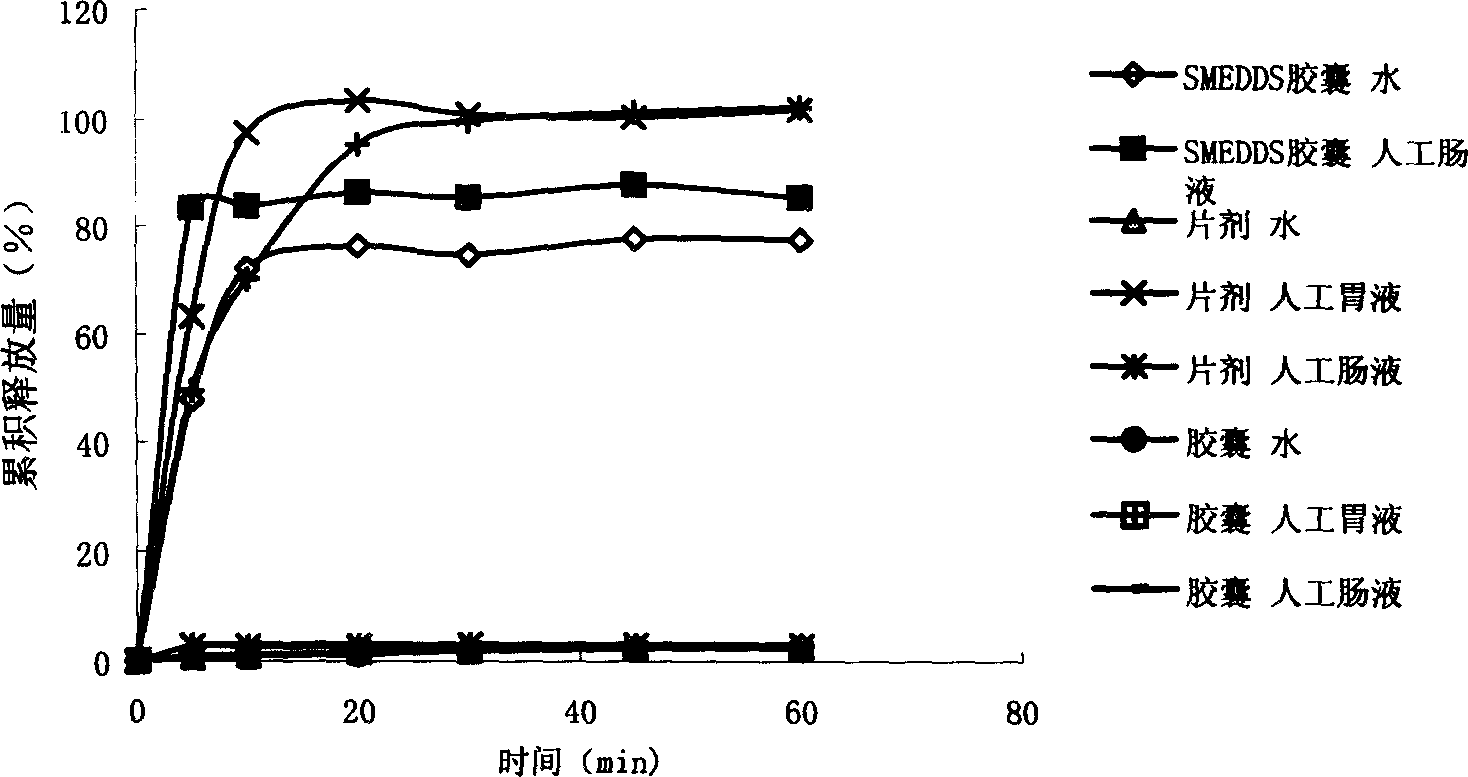
[0041] The cinnarizine self-microemulsifying capsules prepared according to Example 1 were compared with commercially available tablets and capsule dissolution rates: the prepared homogeneous solution was packed into hard capsules according to the prescription amount, and the commercially available cinnar...
Embodiment 2
[0045] The composition of the prescription is as follows: (g)
[0046] Cinnarizine 25
[0047] Dimethylisosorbide 130
[0048] Oleic acid 55
[0049] Tween 80 185
[0050] Vitamin E 5
[0051] Made 1000 softgels in total
[0052] The preparation process is the same as Example 1.
Embodiment 3
[0054] The composition of the prescription is as follows: (g)
[0055] Cinnarizine 25
[0056] Dimethylisosorbide 150
[0057] Oleic acid 38
[0058] Tween 80 190
[0059] Vitamin E 5
[0060] Made 1000 softgels in total
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com