Location controlled releasing method for ultrasonic microbubble contrast medium
A technology of ultrasonic microbubbles and contrast agents, applied in echo/ultrasonic imaging agents, pharmaceutical formulations, hypodermic injection devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0026] Example 1: Synthesis of Microbubble Contrast Agents of the Invention for Adhesion of Genes
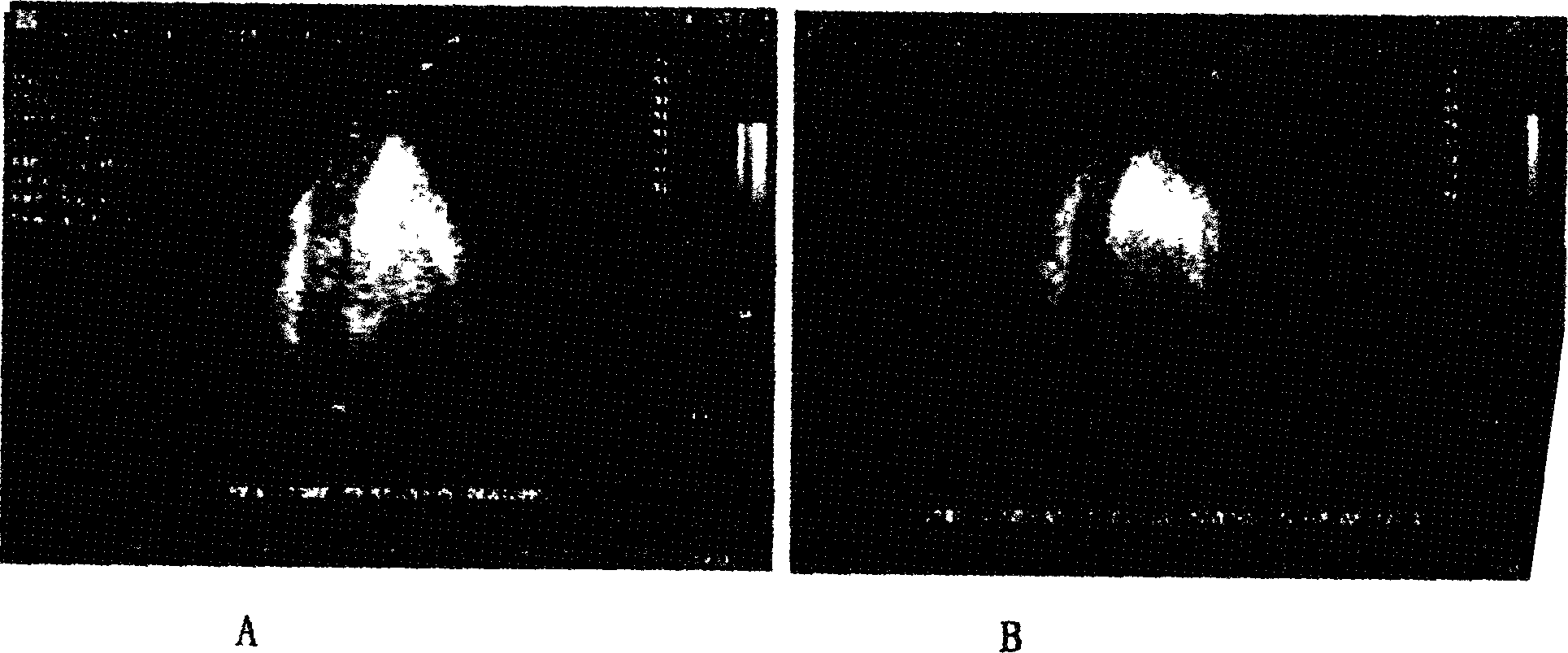
[0027] Lecithin, cholesterol, polyethylene glycol stearyl ethanolamine are dissolved in chloroform in a ratio of 1:3:3 by weight and number, and evaporated in a rotary vacuum to form a film; add 0.9% sodium chloride solution, propylene glycol and glycerol (0.9 % sodium chloride solution: propylene glycol: glycerin=8:1:1), wash the membrane by shaking to form a liposome suspension. Freeze overnight. After thawing, vibrate for 80s with a vibrator at 30% of the maximum output power, and at the same time slowly fill 0.6ml of perfluoropropane gas below through a three-way tube to form lipid fluorocarbon microbubbles. Use a microporous membrane to filter out microbubbles with larger particle sizes. The concentration of lipid microbubble contrast agent was determined by Kool particle counter, and the particle size was determined by microscope. The measured microbubble size is 2-4um,...
example 2
[0028] Example 2: Detection of Gene Adherence to Microbubble Contrast Agents
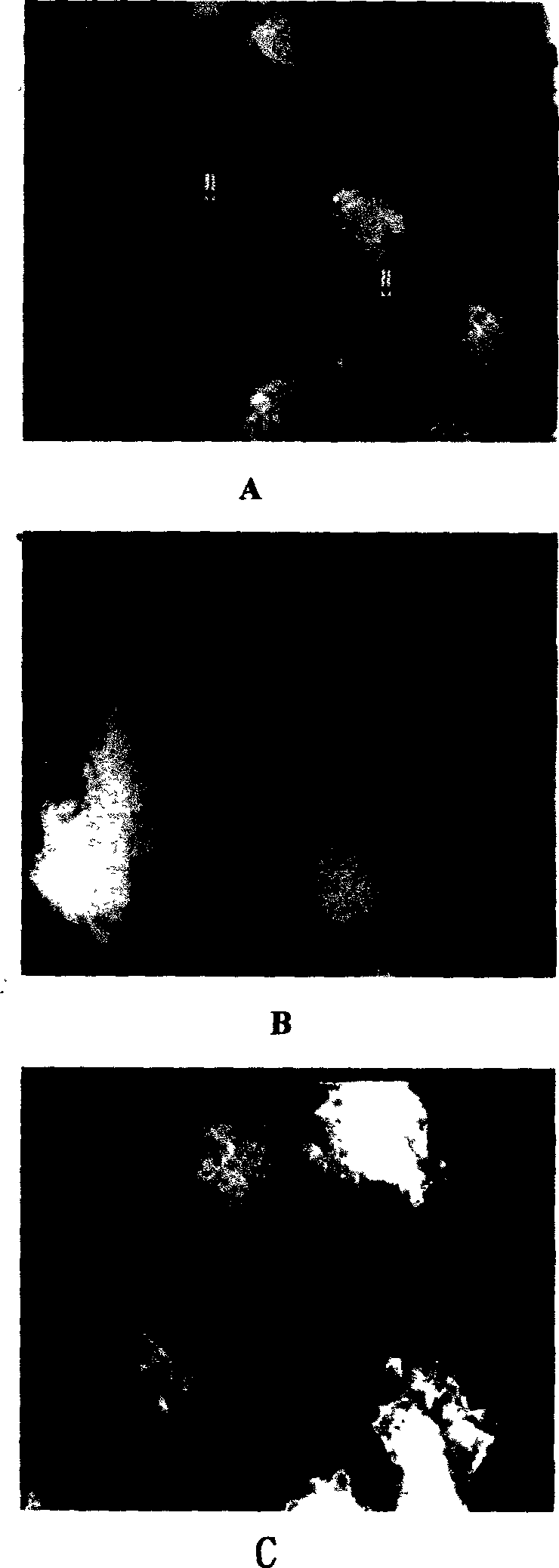
[0029] The lipid shell of the microbubble contrast agent synthesized in Example 1 was fluorescently labeled, and the genes were stained with propidium iodide (PI). Mix 3ml of microbubble contrast agent with 1ml of gene at 4°C for 2 hours, and detect the number of microbubbles emitting two kinds of fluorescence by immunofluorescence method, which is the number of microbubbles adhered to the gene.
example 3
[0030] Example 3: Production of gene encapsulation in microbubble contrast agent
[0031] Mix perfluoropropane liquid and green fluorescent protein gene at a volume ratio of 3:1 at 4°C to form a mixture of fluorocarbon liquid and gene, using lipid materials lecithin, cholesterol, polyethylene glycol stearyl ethanolamine Dissolve with chloroform at a ratio of 1:3:3 by weight and number of parts, and then evaporate in a rotary vacuum to form a film; add an appropriate amount of aqueous phase solvent in proportion: the volume and number of parts is 0.9% sodium chloride solution: propylene glycol: glycerol=8:1: 1 Oscillate to wash the membrane to form a liposome suspension; freeze overnight; after thawing, vibrate with a sonicator at 35% of the maximum output power for an appropriate time of 120s, and at the same time slowly pour in the fluorocarbon liquid and About 6ml of the gene mixture forms a liposome fluorocarbon contrast agent (the outer shell is a liposome, and the solid f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com