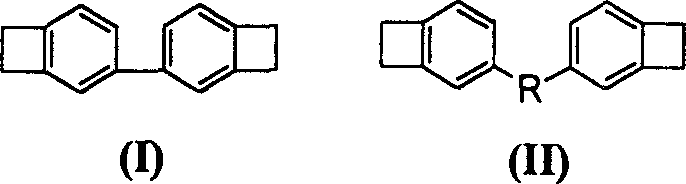
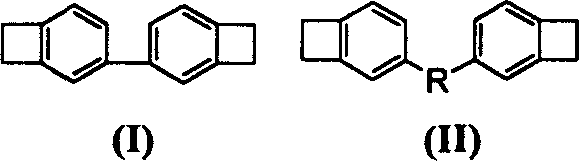
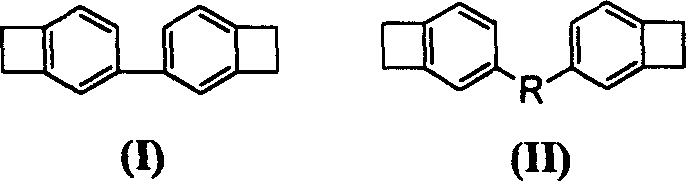
Full conjugation type monomer of dibenzo cyclobutene and preparation method
A technology of bisbenzocyclobutene and benzocyclobutene is applied to the new fully conjugated bisbenzocyclobutene monomer and the field of preparation thereof, and can solve the problems of low yield, unfavorable environmental protection and toxicity. Large and other problems, to achieve high yield, simple post-processing, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In this example, monomer 4,4'-bisbenzocyclobutene was prepared by reaction under nitrogen atmosphere.
[0029] In a 500ml two-necked flask equipped with a magnetic stirring bar, first add 21mmol benzocyclobutene-4-boronic acid ethylene glycol 3.66g, 0.42mmol PdCl 2 75 mg, 1.05 mmol Bu 4 NBr 340mg, 20.4mmol 4-bromobenzocyclobutene 3.70g, 21mmol Na 2 B 4 o 7 10H 2 0.8.01g and absolute ethanol 300ml, then connect the nitrogen conduit and the condensation tube, after the logical nitrogen exhausted the air in the reaction flask, stirred at room temperature for 4 hours; the reaction solution was poured into 300ml water and mixed, and the mixed solution was extracted with sherwood oil ( 4×500ml), combined organic phase, anhydrous Na 2 SO 4 Dry, filter the organic phase with silica gel, rinse with petroleum ether, and concentrate the eluent to dryness under reduced pressure to obtain a colorless oily liquid. Recrystallize from petroleum ether to obtain 4,4'-bisbenzocyclo...
Embodiment 2
[0031] In this example, the monomer 4,4'-bisbenzocyclobutene was prepared by reacting in an air atmosphere.
[0032] In a 500ml round bottom flask equipped with a magnetic stirring bar, first add 21mmol benzocyclobutene-4-boronic acid 3.10g, 0.42mmol Pd(OAc) 2 95mg, 1.05mmolBu 4 NBr 340mg, 42mmol K 3 PO 4 ·3H 2 O 11.20g and absolute ethanol 300ml, stirred at room temperature in the air atmosphere for 24 hours; then the reaction solution was poured into 300ml water and mixed, and the mixed solution was extracted with petroleum ether (4 × 500ml); the organic phases were combined, anhydrous Na 2 SO 4 Dry, filter the organic phase with silica gel, rinse with petroleum ether, and concentrate the eluent to dryness under reduced pressure to obtain a colorless oily liquid. Recrystallize from petroleum ether to obtain 4,4'-bisbenzocyclobutene colorless transparent granular crystals 1.84 g, yield 85%.
Embodiment 3
[0034] In this example, the monomer 4,4'-bisbenzocyclobutene was prepared by reacting in an air atmosphere.
[0035] In a 500ml round bottom flask equipped with a magnetic stirring bar, first add 21mmol benzocyclobutene-4-diisopropyl borate 4.87g, 1.68mmol PdCl 2 298 mg, 2.10 mmol Bu 4 NCl 583mg, 16.2mmol 4-bromobenzocyclobutene 2.95g, 21mmol Na 2 CO 3 2.26g and 500ml of absolute ethanol, stirred at room temperature in an air atmosphere for 48 hours; then the reaction solution was poured into 500ml of water and mixed, and the mixed solution was extracted with petroleum ether (4 × 500ml); the organic phases were combined, anhydrous Na 2 SO 4 Dry, filter the organic phase with silica gel, rinse with petroleum ether, and concentrate the eluent to dryness under reduced pressure to obtain a colorless oily liquid. Recrystallize from petroleum ether to obtain 4,4'-bisbenzocyclobutene colorless transparent granular crystals 4.06 g, Yield >98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com