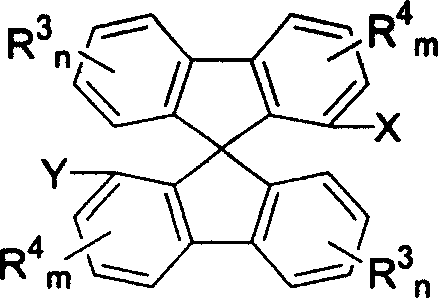
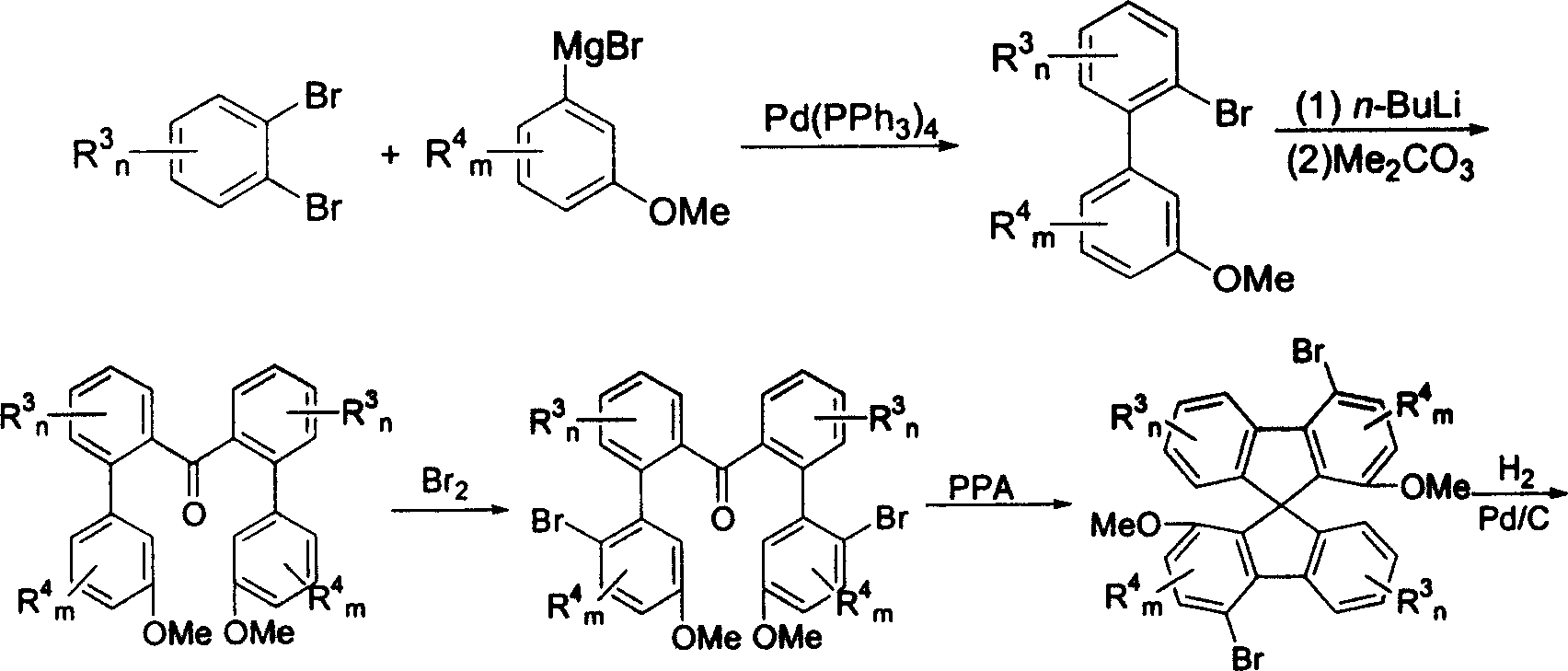
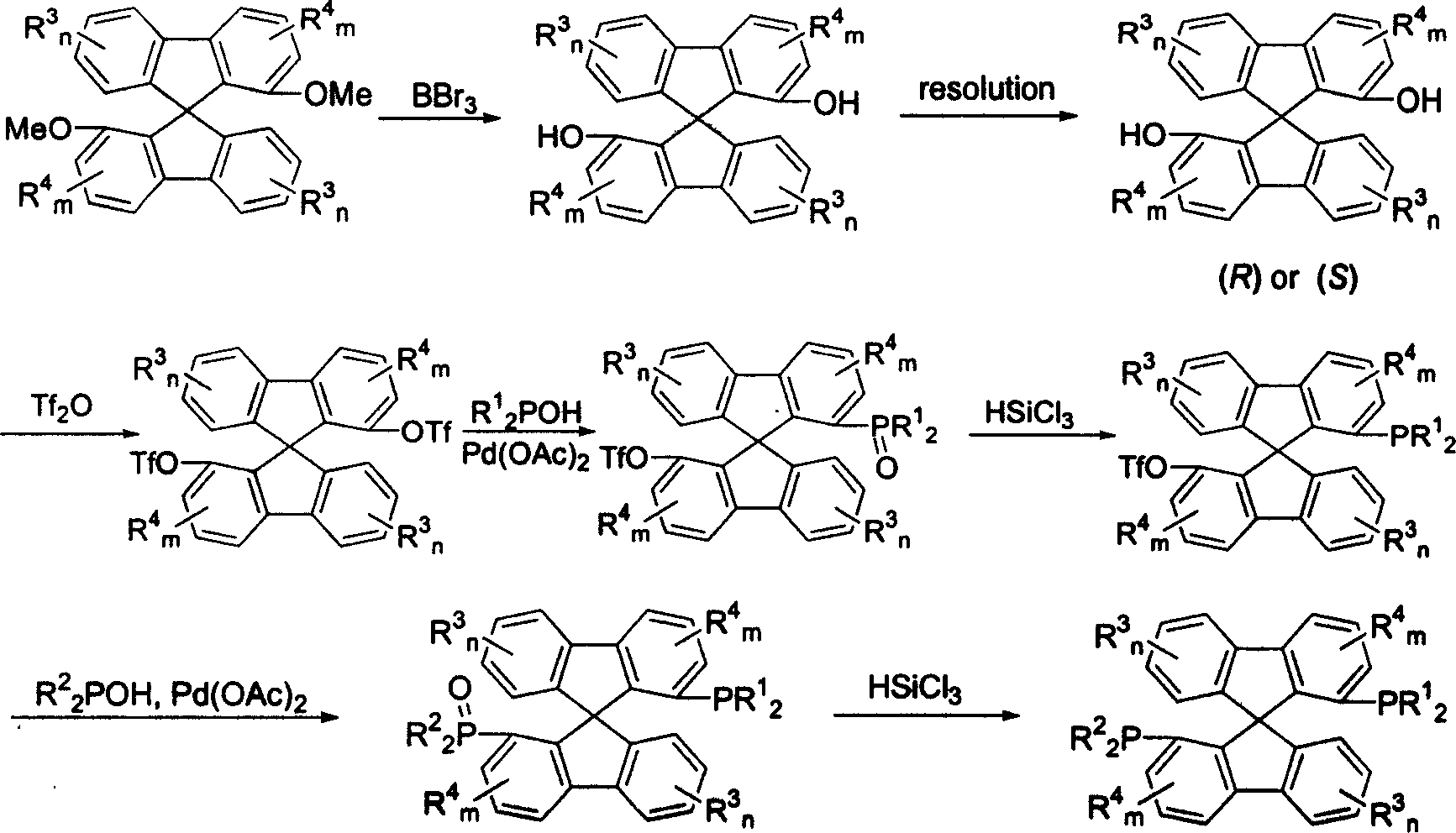
New type spirocyclic diphosphine ligand, and application in asymmetric catalytic hydrogenation
A bisphosphine ligand and spiro ring technology, applied in the field of new spiro ring bisphosphine ligand and its application in asymmetric catalytic hydrogenation, can solve the problems of low conversion number and enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of 2-bromo-3'-methoxy-1,1'-biphenyl
[0039]
[0040] Under a nitrogen atmosphere, 20 mL THF solution of 3-bromoanisole (16.6 g, 89 mmol) was dropped into 80 mL THF suspension of magnesium chips (2.14 g, 89 mmol), while heating to 55 ° C to initiate the reaction, when the reaction occurred Finally, use a water bath to keep the reaction temperature at about 30°C and add the remaining THF solution of 3-bromoanisole dropwise under temperature control. A reaction flask filled with o-dibromobenzene (20g, 92mmol), tetrakis(triphenylphosphine)palladium (1.5g, 1.3mmol) and 100mL THF. After the dropwise addition, the temperature was controlled at 40° C. for 40 hours. Then, most of the solvent was evaporated under reduced pressure, the reaction liquid was diluted with diethyl ether, washed successively with 3N HCl solution and saturated brine, dried over anhydrous magnesium sulfate, filtered, precipitated under reduced pressure, and dissolved in petrole...
Embodiment 2
[0041] Embodiment 2: Preparation of 2,2'-bis-(3-methoxyphenyl)benzophenone
[0042]
[0043] Under nitrogen atmosphere, n-butyllithium (22mL, 2.0M n-hexane solution, 44mmol) was dropped into the pre-cooled to -78°C and contained 2-bromo-3'-methoxy-1,1'-biphenyl (11.7g, 44mmol) and 100mL THF in a reaction flask. After the dropwise addition, continue to stir at -78°C for 20 minutes, then continue to dropwise add a solution of dimethyl carbonate (1.69g, 18.9mmol) dissolved in 25mL THF, after the dropwise addition, naturally warm to -40°C and hold The temperature was maintained for about 3 hours to obtain a yellow suspension. After naturally rising to room temperature, the solution was removed in vacuo, diluted with dichloromethane, washed with 3N HCl solution, dried over anhydrous magnesium sulfate, filtered, and removed under reduced pressure to obtain a solid, acetic acid Ethyl ester was recrystallized to obtain 5.42 g of white solid. Yield: 70% Melting point: 159-160°C. 1...
Embodiment 3
[0044] Example 3: Preparation of 2,2'-bis-(2-bromo-5-methoxyphenyl)-benzophenone
[0045]
[0046] 2,2'-bis-(3-methoxyphenyl)-benzophenone (10g, 25.4mmol) was dissolved in 100mL of dichloromethane, and kept at low temperature with an ice-water bath, then liquid bromine (8.1 g, 50.8 mmol) in 20 mL of dichloromethane solution and stirred at room temperature for 6 hours, the reaction solution was diluted with dichloromethane, and the organic phase was washed successively with water-saturated sodium bisulfite and saturated aqueous sodium bicarbonate until neutral, sulfuric acid Dried over magnesium, filtered, and precipitated under reduced pressure to obtain 14.2 g of light yellow solid. Yield: 100% Melting point: 158-160°C. 1H NMR (300MHz, CDCl 3 ): 3.69 (s, 3H, Ar-OCH3), 3.37 (s, 3H, Ar-OCH3), 6.63-6.69 (m, 4H, Ar-H), 7.19-7.21 (m, 4H, Ar-H), 7.34-7.50 (m, 5H, Ar-H), 7.59 (d, 1H, J=7.8Hz, Ar-H); 13 C NMR (75 MHz, CDCl 3 ): δ55.4, 113.7, 113.8, 114.4, 115.3, 116.5, 127.2, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com