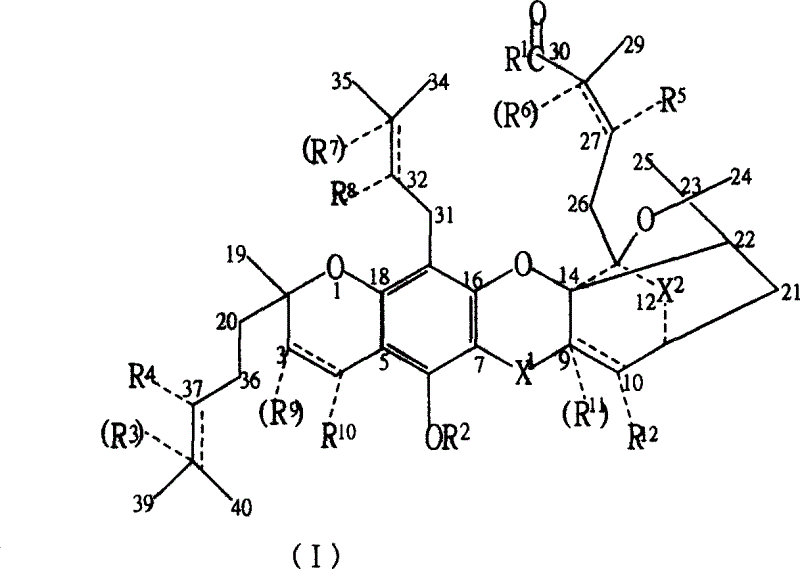
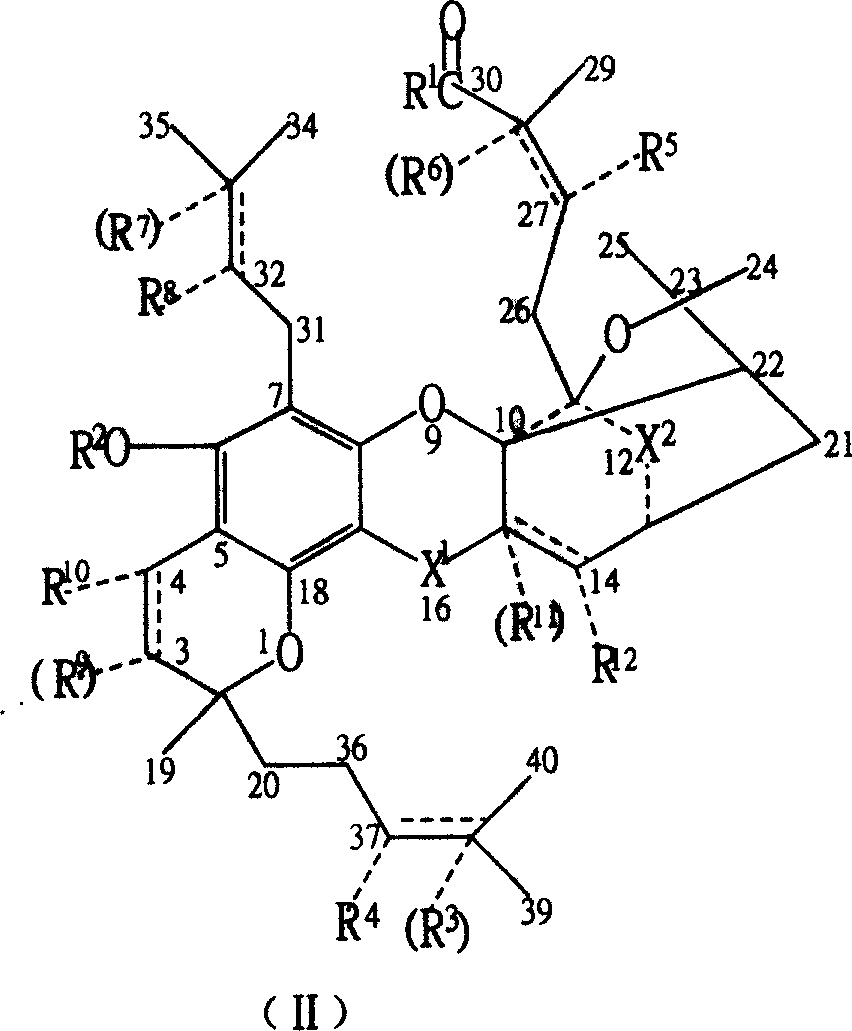
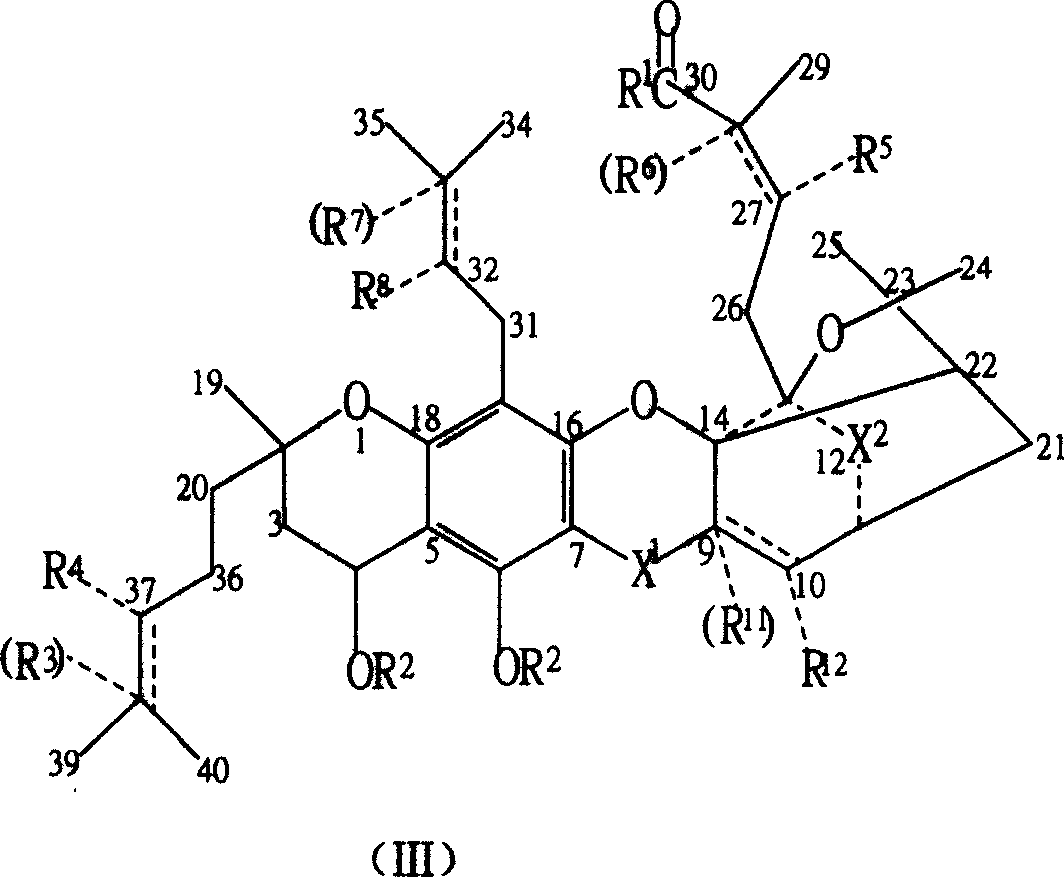
New compound ramification of garcinia acid
A new technology of gambogic acid and derivatives, applied in the direction of active ingredients of nitro compounds, organic chemistry, etc., can solve problems such as low stability, insolubility, and instability of gambogic acid, and achieve good distribution coefficient and low toxicity Side effects, highly stable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of gambogic acid ketone carbonyl reduction
[0062]In a 250 ml three-neck flask with stirring, a 0-50°C thermometer and a 25 ml constant pressure dropping funnel, add 6.47 grams of gambogic acid (0.01 mol, 97%), 60 ml of methanol, and 0.42 grams of lithium aluminum hydride (0.011 mol, 98%) was dissolved in a constant-pressure dropping funnel with 10 ml of diethyl ether, the stirring was started, and the lithium aluminum hydride solution was slowly added dropwise while controlling the temperature of the methanol solution at 0-5°C. After the dropwise addition was completed, the stirring reaction was continued for more than 3 hours. Then the reactant was poured into 60 g of ice-water mixture while being stirred with a glass rod, extracted three times with 70 ml of ether (30, 30, 10 ml), and the ether solutions were combined. The ether was washed with water until it was neutral, the aqueous phase was discarded, the organic phase was dried over anhydro...
Embodiment 2
[0067] Embodiment two gambogic acid 20 Preparation of C esters
[0068] In a 250 ml three-neck flask with stirring and a thermometer at 0-50°C, add 6.47 grams of gambogic acid (0.01 moles, 97%), add 70 milliliters of ethanol, and stir and dissolve 2.5 grams of DMAP (0.02 moles) completely. Add a small amount of anhydrous magnesium sulfate and stir for 3 hours, remove magnesium sulfate by filtration, 2.5 grams (0.02 moles) of DMAP and 1.8 grams (0.02 moles) of EDC, stir and react at room temperature for more than 2 hours, and pour the reactant into ice water , extract the target product in the decantate three times (40, 30, 10) with 80 milliliters of ether, discard the water phase, combine the ether phase, remove the organic solvent after drying with anhydrous sodium sulfate, and the crude product obtained is separated with a silica gel column , obtain the higher gambogic acid ester of purity, promptly obtain the R in the general formula (I) 1 for CH 3 CH 2 O- gambogic acid...
Embodiment 3
[0075] Example three gambogic acid 30 Preparation of C amides
[0076] In a 250 ml three-neck flask with stirring and a 0-50° C. thermometer, add 50 ml of acetonitrile and 6.47 g of gambogic acid (0.01 mol, 97%). After stirring to dissolve completely, add a small amount of anhydrous magnesium sulfate and stir for 3 hours, then filter to remove magnesium sulfate. Add 1.45 g (0.02 mol) of butylamine, 2.5 g (0.02 mol) of DMAP and 1.8 g (0.02 mol) of EDC, and stir the reaction at room temperature for more than 6 hours. The reactant was poured into ice water, and the target product in the decantate was extracted three times (40, 30, 10) with 80 ml of ether, the water phase was discarded, the ether phase was combined, and after drying with anhydrous sodium sulfate, the organic solvent was removed. The obtained crude product is separated with a silica gel column to obtain gambogic acid butyramide with higher purity. Promptly obtain the R in the general formula (I) 1 for CH 3 (CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com