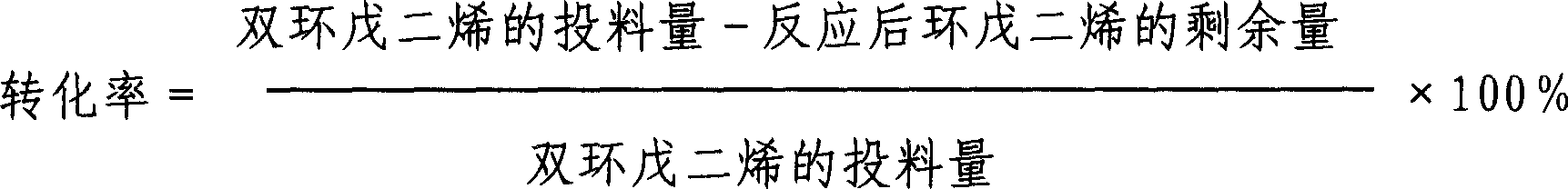Method for preparing 2,5-norbornadiene from dicyclo pentadiene
A technology of dicyclopentadiene and norbornol, which is applied in the directions of isomerization to hydrocarbon production, organic chemistry, etc., can solve the problems of high production cost and low yield, and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9
[0016] A stirring device and a cooling coil were installed in a 2-liter autoclave, an electric heating device was installed outside the autoclave, and a tubular gas distributor was installed at the bottom of the autoclave. First, the required amount of dicyclopentadiene is dissolved in the required amount of solvent acetone, and the solvent material in which the dicyclopentadiene is dissolved is pumped into the autoclave. After the air in the kettle was replaced with nitrogen three times, the acetylene was slowly passed into the solvent through a tubular gas distributor, and the required feeding amount of acetylene was controlled by a subtractive weighing method. Start stirring and heat up to the required reaction temperature for reaction. After 3 to 7 hours of reaction, cool the kettle down to room temperature with cooling water to stop the reaction.
[0017] After the reaction, the reaction materials are moved into a rectification tower with 15 theoretical plates, and rectif...
Embodiment 10
[0019] The solvent was changed to dimethyl sulfoxide, and the rest were the same as in Examples 1-9.
[0020] The specific reaction conditions of each embodiment are shown in Table 1, and the reaction results are shown in Table 2.
[0021] temperature reflex
[0022] Dicyclopentadiene
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com