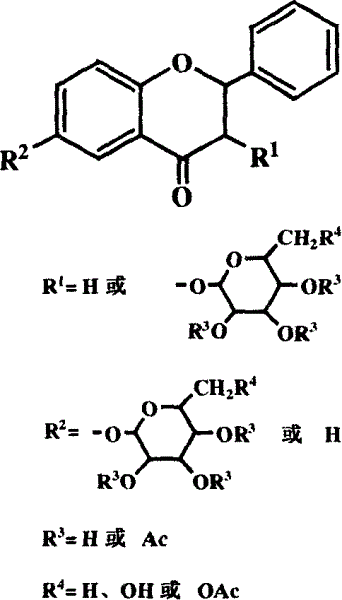
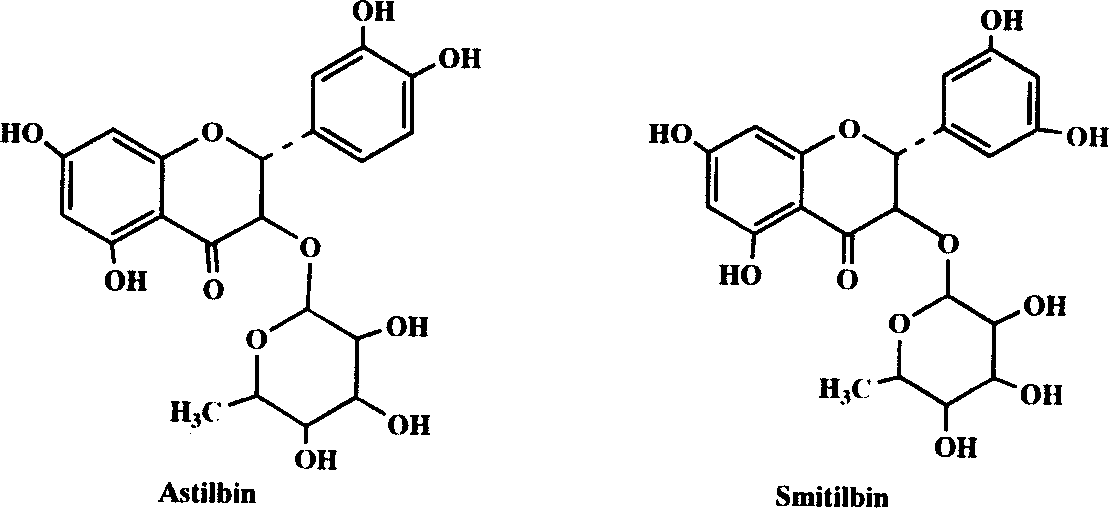
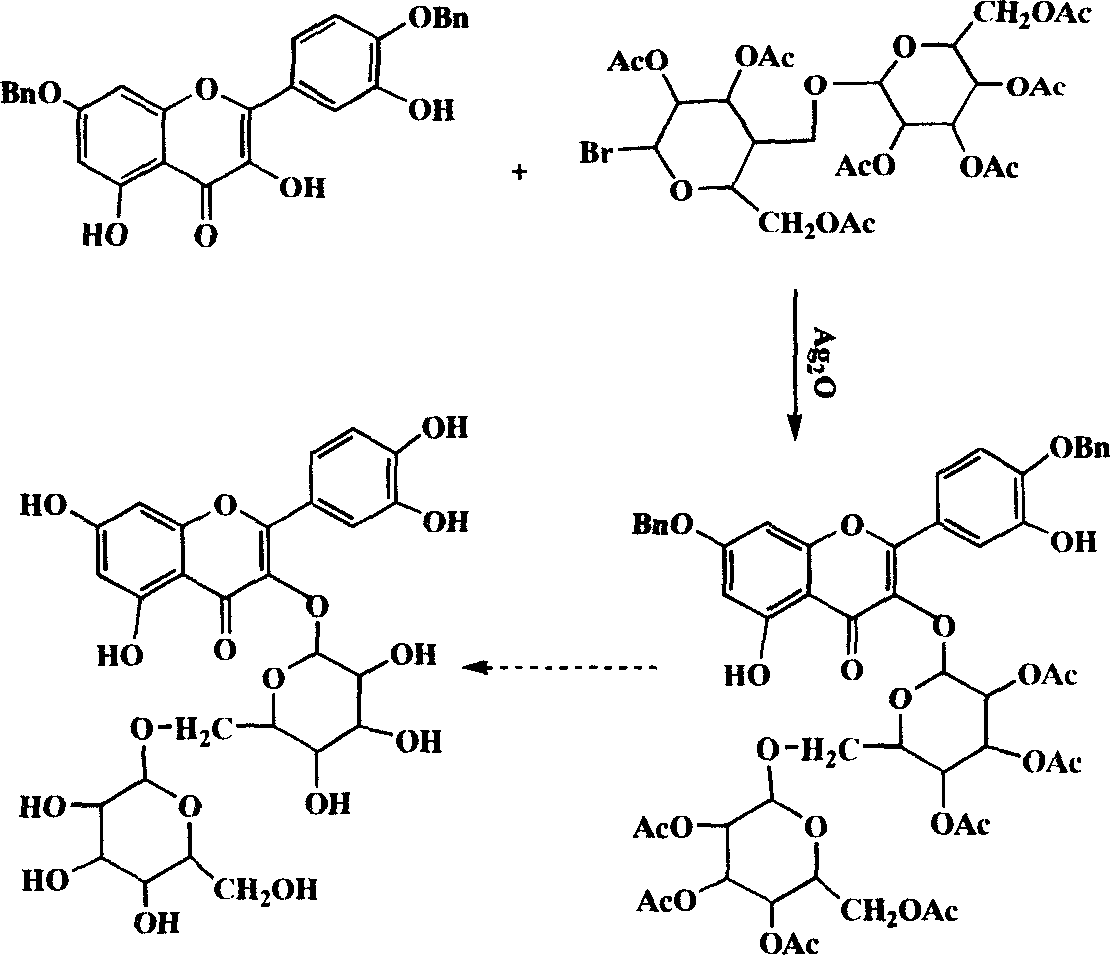
Flavanone glycoside and its preparation and use
A flavanone and glycoside technology, which is applied in the directions of sugar derivatives, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of difficult processing, many reaction steps, and long reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, the preparation of 6-L-rhamnopyranosyl flavanone (IV)
[0027]
[0028] 1. Preparation of 6-(2,3,4-triacetyl-L-rhamnopyranosyl)flavanone (III):
[0029] 6-Hydroxyflavanone (I) (0.484g, 2.017mmol) and 2,3,4-triacetyl-1-oxo-L-rhamnopyranosyltrichloroethylimine (II) (0.884g , 2.035mmol) were mixed, and after vacuum drying for 2h, 54ml of anhydrous CH was added to the mixture 2 Cl 2 , the system was cooled to -42°C in a liquid nitrogen-ethanol bath, N 2 Under protection, 46 μl of trimethylsilyl trifluoromethanesulfonate was added dropwise, and after the addition was completed, the reaction system gradually returned to room temperature, and the total reaction time was about 3 hours. 3: 2) After monitoring the reaction, triethylamine was added to the system to terminate the reaction, and the solvent was removed by a rotary evaporator to obtain a brownish-yellow viscous liquid, which was separated and purified by column chromatography (eluent: petroleum eth...
Embodiment 2
[0048] Example 2, Preparation of 6-D-glucopyranosylflavanone (VII)
[0049]
[0050] 1. Preparation of 6-(2,3,4,6-tetraacetyl-D-glucopyranosyl)flavanone (VI):
[0051] 6-Hydroxyflavanone (I) (0.507g, 2.113mmol) and 2,3,4,6-tetraacetyl-1-oxo-D-glucopyranosyl trichloroethylimine (V) (1.040 g, 2.111mmol) were mixed, and after vacuum drying for 2h, 70ml of anhydrous CH was added to the mixture 2 Cl 2 , the system was cooled to -78°C in a liquid nitrogen-ethanol bath, N 2 Under protection, 45 μl of trimethylsilyl trifluoromethanesulfonate was added dropwise. After the addition was complete, the reaction system returned to room temperature gradually. 3: 2) After monitoring the reaction, triethylamine was added to the system to terminate the reaction, and the solvent was removed by a rotary evaporator to obtain a brownish-yellow viscous liquid, which was separated and purified by column chromatography (the eluent was petroleum ether: ethyl acetate =3:1) to obtain 0.610 g of li...
Embodiment 3
[0070] Example 3, Preparation of 3-L-rhamnopyranosylflavanone (XII)
[0071]
[0072] The preparation of 1.3-hydroxyl-4-flavanone dimethyl acetal (IX):
[0073] Flavanone (VIII) (4.48g, 0.02mol) was mixed with 100ml of absolute anhydrous methanol, cooled to 0°C, and a mixed solution of potassium hydroxide (3.36g, 0.06mol) and 50ml of methanol was added dropwise, the solution was brownish yellow, Add in batches (4 to 5 parts) iodobenzene diacetate (C 6 h 5 IO(Ac) 2 , 7.0 g, 0.02 mol), for about 5 minutes, the mixture was stirred at 0° C. for 1 hour, then the ice bath was removed, and stirred overnight at room temperature. After methanol was removed by rotary evaporation, 100ml of water and potassium carbonate were added to the system for neutralization, and extracted 5 times with ether, about 40ml each time. The organic phases were combined and dried over anhydrous magnesium sulfate. After removing the desiccant by filtration, the solution was concentrated to give a colo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com