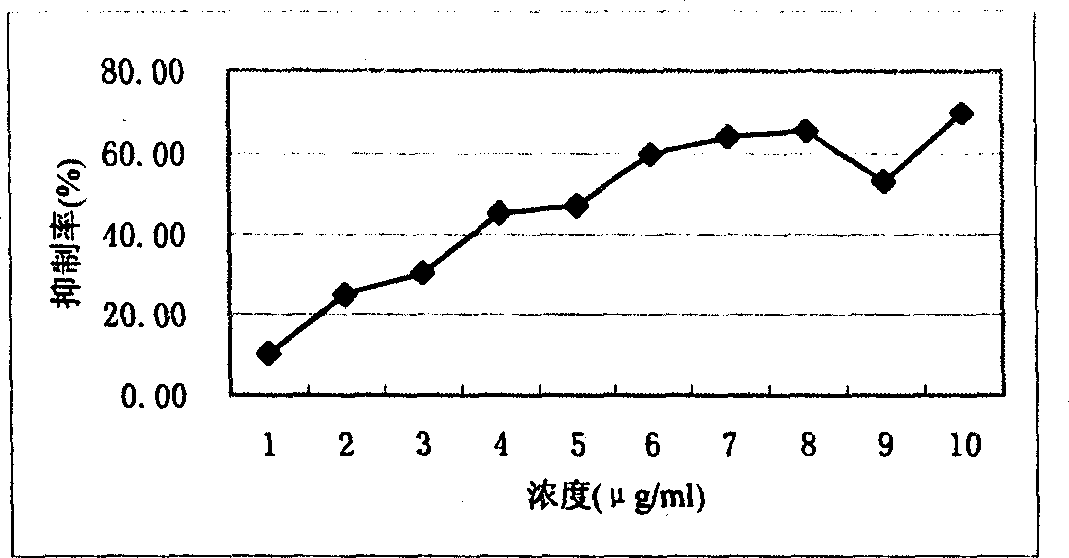
Cyanoacrylate derivatives and their preparation method and biological activity
A technology of cyanoacrylic acid and its derivatives, which can be used in botany equipment and methods, organic active ingredients, biocides, etc., and can solve problems such as activity decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, the synthesis of 3-p-trifluoromethylaniline-3-methylthio-2-cyanoacrylate (the compound number is a):
[0049] (1) Synthesis of 3,3-dimethylthio-2-cyanoacrylate:
[0050] After adding 400mL of absolute ethanol, 34.0g (0.301mol) of ethyl cyanoacetate, and 23.0g (0.303mol) of carbon disulfide into a 1000mL four-necked bottle, the temperature was controlled to be less than 20°C, and 360mL of sodium ethoxide (0.610M) with a concentration of 1.7M was added dropwise. mol), the dropwise addition time is about 1h, after the dropwise addition is completed, stir at room temperature for 8h, then add dropwise 38.5g dimethyl sulfate (0.305mol), dropwisely complete in 30min, heat to reflux for 2.5h, and the reaction is complete. The reaction system was poured into 500mL of water and allowed to stand overnight to obtain a white needle-like solid, which was suction filtered and dried. Recrystallized twice with n-hexane: ether (1:1) to obtain 36.7 g of white crystals, name...
Embodiment 2
[0054]Example 2, Synthesis of 3-(2,6-dichloro-4-trifluoromethyl)aniline-3-methylthio-2-cyanoacrylate (compound number is b):
[0055] (1) Synthesis of 3,3-dimethylthio-2-cyanoacrylate,
[0056] Synthesize as in Example 1 (1) method and conditions.
[0057] (2) Synthesis of 3-(2,6-dichloro-4-trifluoromethyl)aniline-3-methylthio-2-cyanoacrylate:
[0058] 2.17g (0.01mol) of 3,3-dimethylthio-2-cyanoacrylate, 2.30g (0.01mol) of 2,6-dichloro-4-trifluoromethylaniline were added to a 50mL three-necked flask, and 0.80 60% NaH (0.02mol) was added, then 20mL of DMF and 20mL of toluene mixed solvent was added dropwise, the temperature of the addition was controlled below 10°C, and after stirring at room temperature for 40h, the reaction was complete. Pour the reaction system into 100 mL of ice water, separate the water phase, slowly adjust the pH with 10% HCl, a light yellow solid precipitates, dry it, and recrystallize it with ethanol to obtain 2.61 g of white crystals, namely 3-(2,6-d...
Embodiment 3
[0060] Example 3, Synthesis of 3-(4-methylbenzothiazole-2-)amine-3-methylthio-2-cyanoacrylate (compound number is d):
[0061] (1) Synthesis of 3,3-dimethylthio-2-cyanoacrylate,
[0062] Synthesize as in Example 1 (1) method and conditions.
[0063] (2) Synthesis of 3-(4-methylbenzothiazole-2-)amine-3-methylthio-2-cyanoacrylate: 3,3-dimethylthio-2-cyanoacrylate 2.65 g (0.012mol), and 2.0g (0.012mol) of 2-amino-4-methylbenzothiazole were added to a 100mL three-necked flask. After adding 0.96g (0.024mol) of 60% NaH, then dropwise add 25mL of toluene and 25mL of DMF as a mixed solvent, use an ice bath to control the dropwise addition temperature not to exceed 20°C, and complete the dropwise addition in about 10min, after stirring at room temperature for 48h, TLC traced the completion of the reaction . The reaction system was poured into 150 mL of ice water, the water phase was separated, and 10% HCl solution was added dropwise, and milky yellow solids were precipitated. When t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com