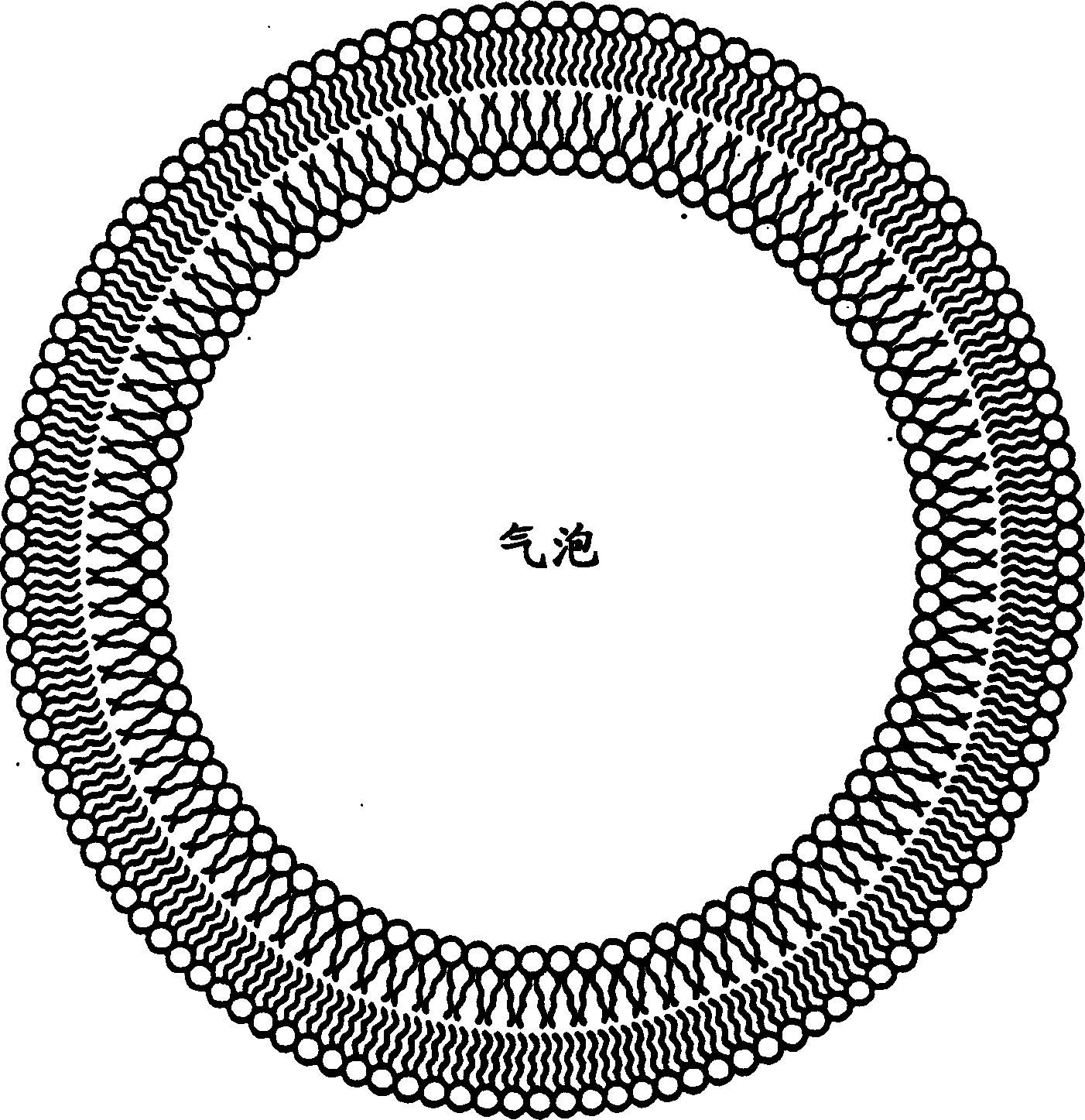
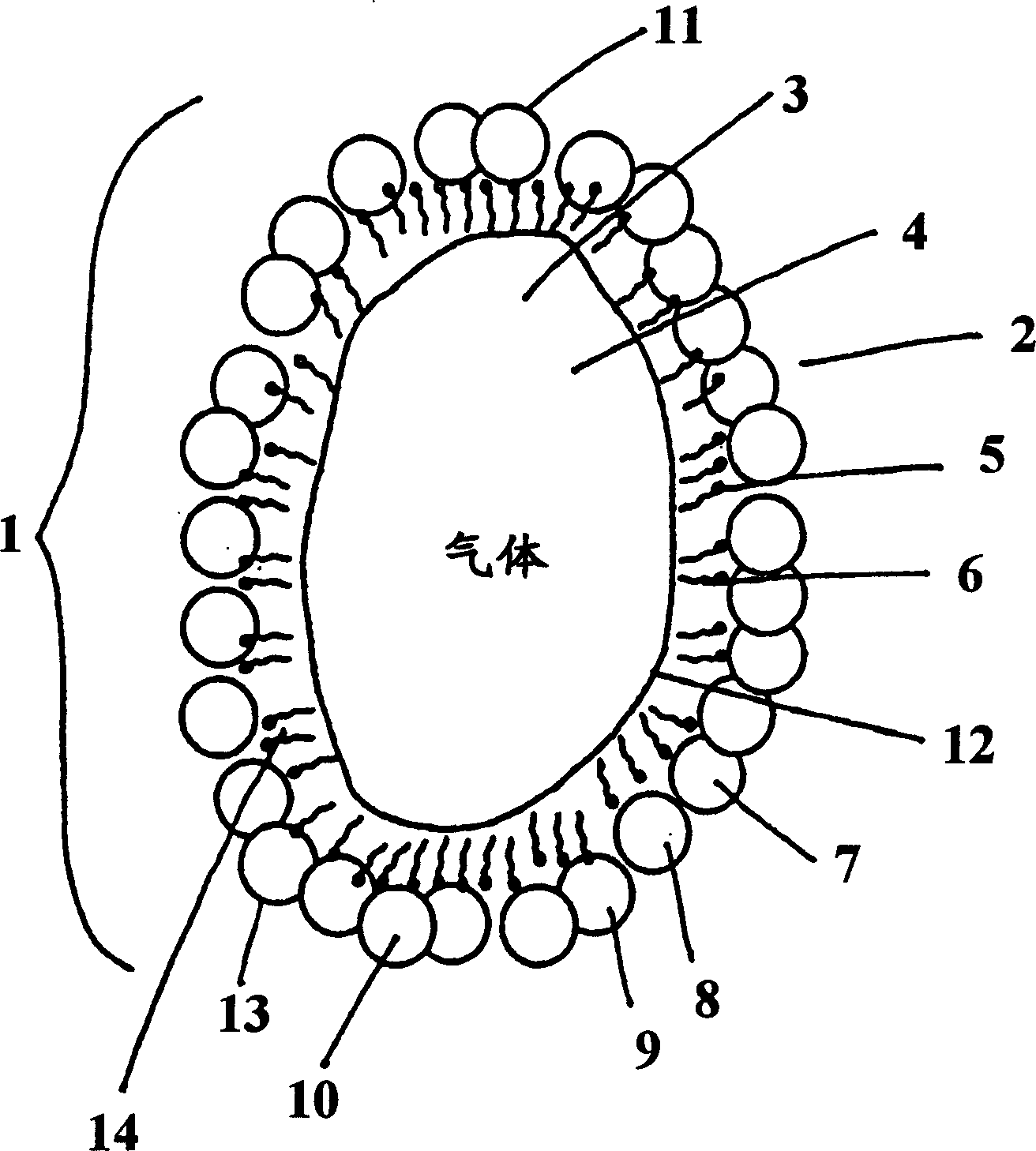
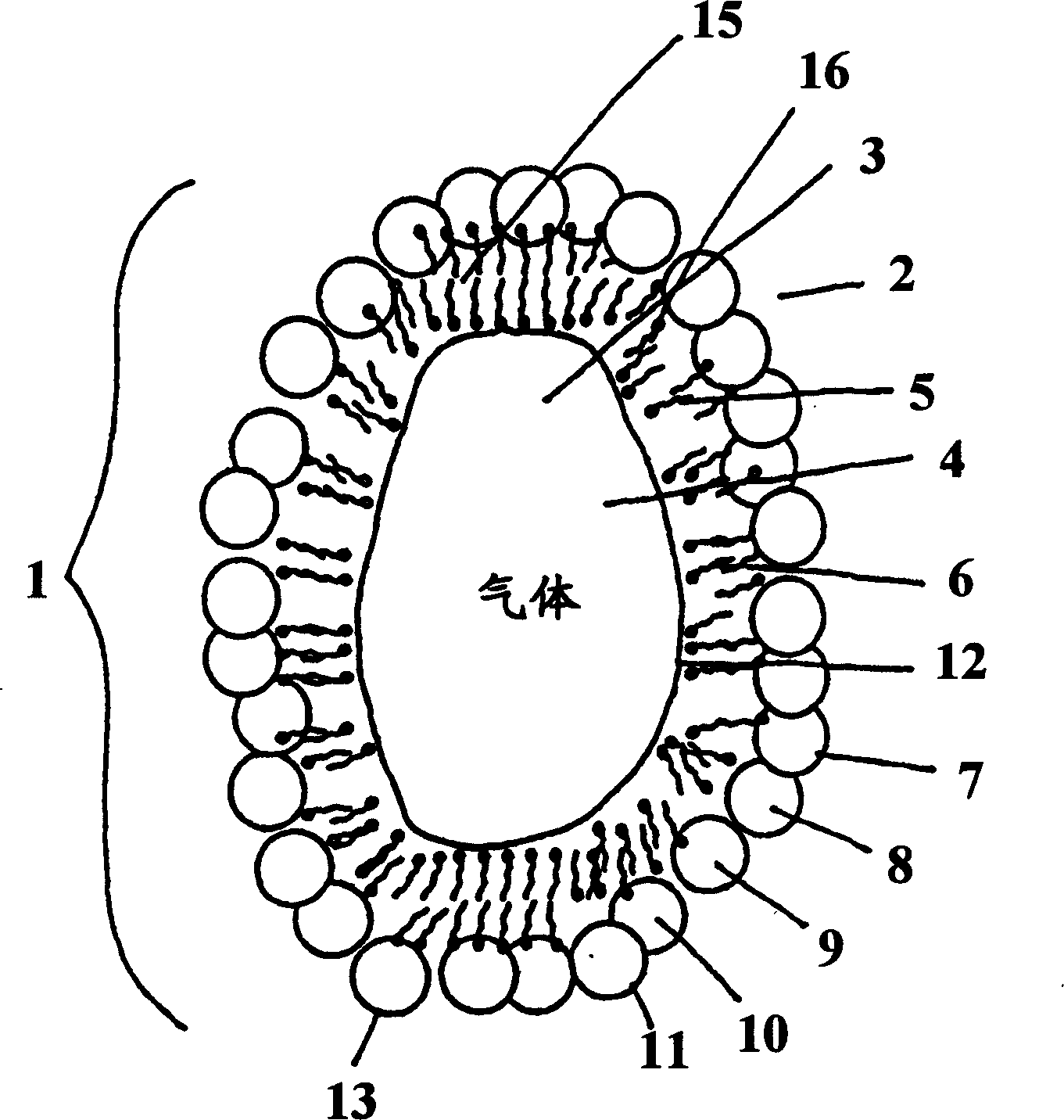
Gas micro-liposome compound
A technology of liposomes and complexes, applied in the directions of liposome delivery, drug combination, peptide/protein composition, etc., can solve the problems of poor ultrasound imaging quality, disappointing diagnostic results, and inability to quantify, and achieve improved contrast, excellent Acoustic response characteristics, the effect of small segregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] A saline glycerol solution (100 ml) was prepared comprising glycerol (10 ml) and NaCl (680±2 mg) dissolved in water (final volume 100 ml). In a 100ml volumetric flask, DPPC (dipalmitoylphosphatidylcholine) (40.0mg), MPEG500 DPPE (dipalmitoylphosphatidylethanolamine) (30.0mg) and DPPA (4.5mg) were mixed with propylene glycol (10ml), and The volumetric flask was placed in a hot water bath (70°C) and sonicated for 15 minutes until the solution was clear. A saline / glycerol solution was then added to bring the mixture to a final volume of 100 ml, and the suspension was mixed well. The suspension (1.6ml) was transferred to a 2ml borosilicate glass vial. The headspace was purged with perfluoropropane gas and the vials were stoppered and sealed. The stoppers were West Gray V 50 lyo 13mm, 4416 / 50 elastic formula. The sealing ring is an aluminum sealing strip that can be torn off gently. Using IONOS Ionomix The vial containing the lipid suspension was shaken for 45 seconds...
Embodiment 2
[0120] Part A. 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-cyclo(arginine-glycine-aspartic acid-D-phenylalanine-lysine)-dodecanoate Synthesis of conjugates
[0121]
[0122] Under stirring (5 minutes), disuccinimidyl dodecanoate (0.424 g, 1 mmol), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) (1.489 g, 1 mmol ) and cyclic (arginine-glycine-aspartic acid-D-phenylalanine-lysine) TFA salt (0.831g, 1mmol) (for the synthesis of the cyclic peptide targeting part, refer to US Patent Series No. 09 / 281,474, the method of which is incorporated herein by reference) was dissolved in chloroform (25 ml). Sodium carbonate (1 mmol) and sodium sulfate (1 mmol) were added and the solution was stirred at room temperature under nitrogen (18 hours). Chloroform was removed in vacuo and the title compound was purified from the crude product mixture by preparative HPLC or recrystallization.
[0123] Part B. Preparation of Control Agent Compositions
[0124] Coupling of 1,2-dipa...
Embodiment 3
[0126] Part A. Omega-Amino-PEG 3400 - Preparation of the ring (arginine-glycine-aspartic acid-D-phenylalanine-lysine):
[0127]
[0128] Add triethylamine (3mmol) to N-Boc-PEG 3400 -Succinimidyl ester (1 mmol) and cyclo(arginine-glycine-aspartate-D-phenylalanine-lysine) (1 mmol) in dimethylformamide (DMF) (25 mL) middle. The reaction mixture was stirred overnight at room temperature under a nitrogen atmosphere and the solvent was removed in vacuo. The crude product was dissolved in trifluoroacetic acid / dichloromethane (1:1 v / v) and stirred for 4 hours. The volatiles were removed and the TFA salt of the title compound was isolated by trituration in diethyl ether.
[0129] Part B: DPPE-PEG 3400 - Preparation of cyclic (arginine-glycine-aspartic acid-D-phenylalanine-lysine)-dodecanoate conjugates:
[0130]
[0131]Disuccinimidyl dodecanoate (1 mmol), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) (1 mmol) and ω-amino-PEG were mixed with stirring for 5 minutes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com