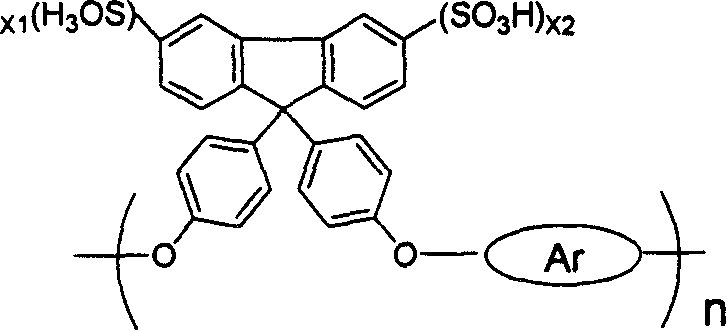
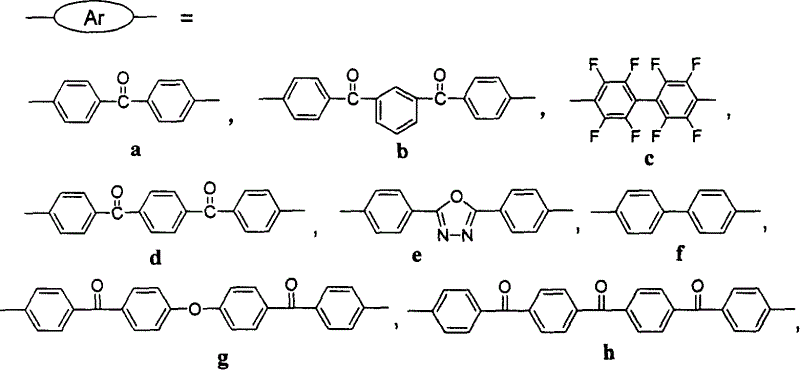
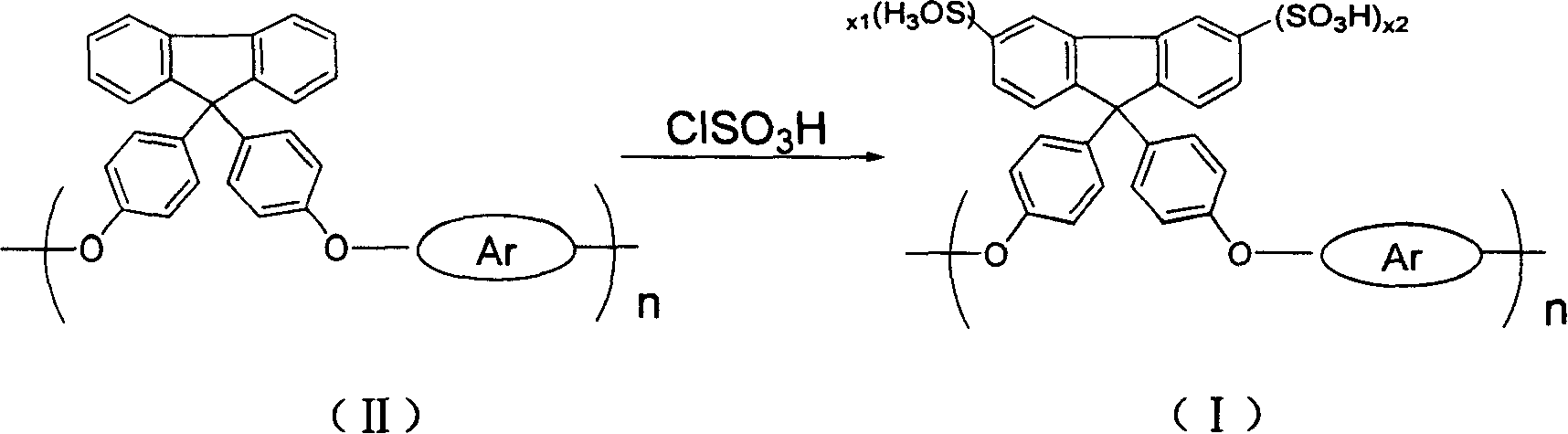
Sulfonated fluorine-containing poly-arylether (arone) and its preparing method and use
A technology for sulfonating fluorene-containing polyarylethers and polyarylethers, which is applied in the field of sulfonated fluorene-containing polyarylethers (ketones) and its preparation and application, and can solve the problems of easy breakage of ether bonds, reduction of chemical stability and service life, etc. problem, to achieve the effect of prolonging service life, improving chemical stability and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0031] Example 1-1 Sulfonation of fluorene-containing polyaryletherketone IIa:
[0032] At room temperature, add 0.25mmol of fluorene-containing polyaryletherketone IIa into a 100mL single-necked flask, add 25mL of dichloromethane, and stir it to fully dissolve it. Measure 0.0665ml (1mmol, 0.117g) of chlorosulfonic acid at a ratio of sulfonic acid molecules of 1:4, add 3mL of dichloromethane to prepare a solution, slowly drop it in, stir and react for 5 hours, and a brown precipitate forms , poured out dichloromethane, washed three times with 5mL of n-hexane each time, added 5mL of DMAc, stirred for 1-2 hours to fully dissolve, added 10mL of 3wt% sodium hydroxide solution, stirred for 6 hours, and then added 100mL of 5wt% HCl solution, reacted for 5 hours, and the reaction product was transferred into a dialysis bag, and was dialyzed with deionized water until the water outside the dialysis bag was neutral, and then evaporated to dryness to obtain the sulfonated fluorene-conta...
Embodiment 1-2
[0033] Embodiment 1-2, 1-3, 1-4, 1-5, 1-6, 1-7, 1-8, 1-9, 1-10 are containing fluorene polyarylether (ketone) IIb~IIj respectively sulfonation, its sulfonation method and steps are the same as in Example 1-1, the difference is that the raw material ratio (the ratio of fluorene structural units to chlorosulfonic acid molecules in the fluorene-containing polyarylether (ketone) molecule) is different, and the obtained The degree of sulfonation of the product (i.e. X 1 +X 2 value) are different, as shown in Table 1:
[0034] Example
[0035] 2. Membrane preparation
Embodiment 2-1
[0036]Example 2-1 Preparation of single-component membrane by sulfonated fluorene-containing polyaryletherketone Ia:
[0037] Take 1 g of the sulfonated fluorene-containing polyaryletherketone Ia prepared according to Example 1-1, add 20 mL of DMAc, stir for 3 hours to dissolve completely, filter, and concentrate the filtrate at 65 ° C until it is estimated that it will not flow out of the glass plate when it is poured on the glass plate Then pour it on a clean glass plate to form a film, put it into a vacuum oven, and evaporate the solvent in a vacuum at a temperature of 40°C to obtain a dry finished single-component film.
[0038] The prepared film was tested with Fenton's reagent to measure its anti-oxidation property, the method is in 30% H 2 o 2 Add 30ppm of FeSO to the solution 4 , at 25°C, put 1cm 2 For the film samples, record the time when the film begins to break down.
[0039] The prepared membrane was acidified with 1M sulfuric acid at 80° C. for 24 hours, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of sulfonation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com