Bicyclic CB2 cannabinoid receptor ligands
A cyclic, dimethyl technology, applied in the field of α-pinene derivatives and pharmaceutical compositions thereof, can solve the problems of unknown therapeutic activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
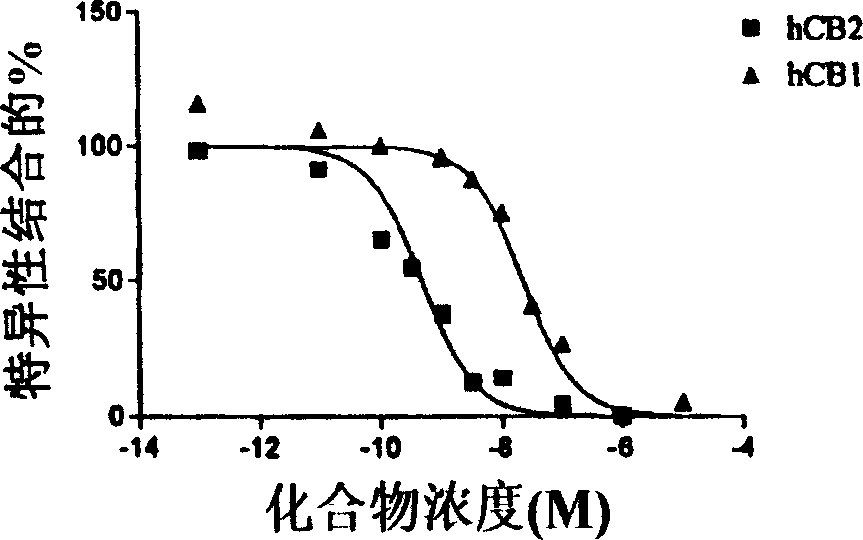
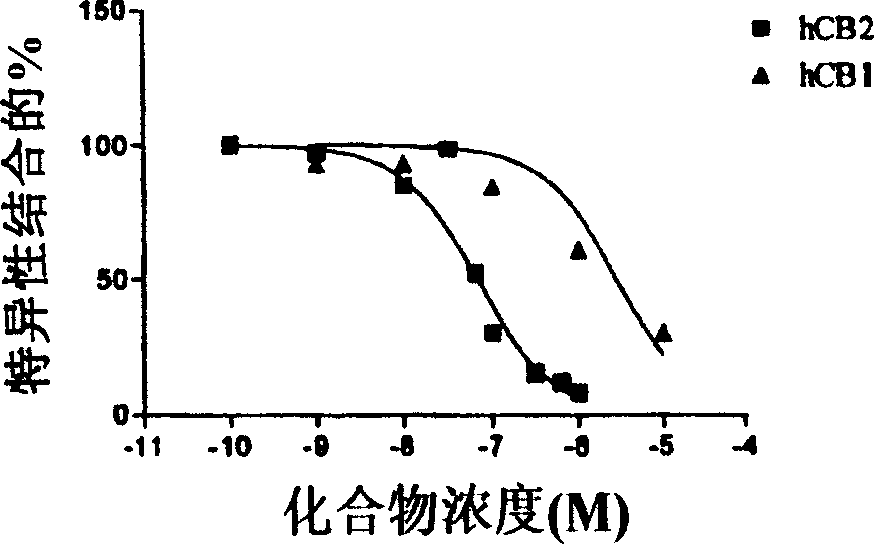
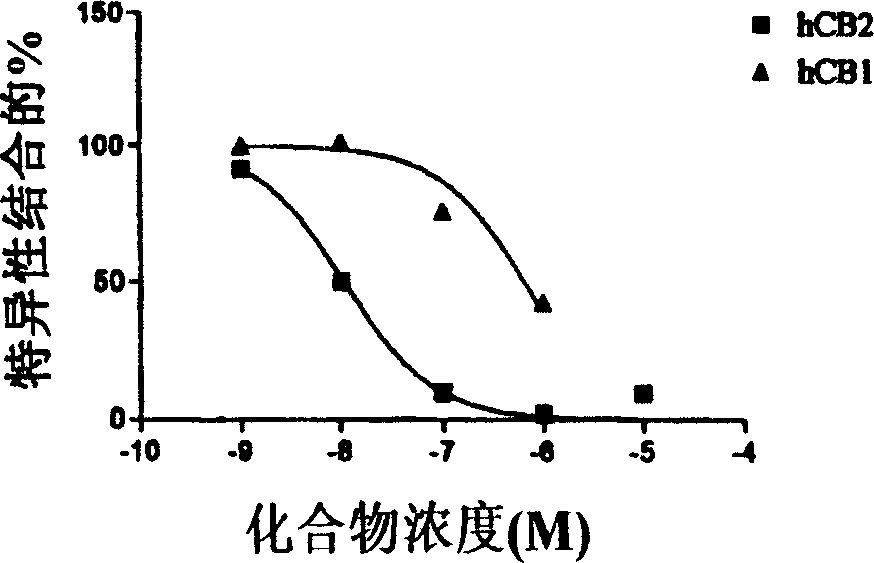
[0339] Binding affinity of CB1 and CB2 receptors.
[0340] Displacement from the CB1 receptor by detecting new compounds on the membrane [ 3 H] The ability of CP55940 to perform binding analysis of CB1, the membrane is derived from HEK-293 cells stably transfected with hCB1 (Perkin Elmer / NEN). Dilute the membrane in assay buffer (50mM Tris-HCl, 2.5mM EDTA, 5mM MgCl 2 , 1mg / ml BSA, pH=7.4) to get 500μg protein / ml. In the presence or absence of the bicyclic test compound, mix 50μl of the diluted membrane (25μg) with [ 3 H] CP55940 was incubated together in a total volume of 0.5 ml. The test compound was dissolved in DMSO and diluted in the assay buffer to obtain a final concentration of 0.1% solvent. Add the same amount of control sample as the medium. Non-specific binding was measured by adding 10 μM WIN 55212-2. After incubating at 30°C for 1.5 hours, the reaction was filtered through a Whatman 934A / H filter (pre-soaked with 0.1% polyethyleneimine (PEI)).
[0341] By detecting the...
Embodiment 2
[0357] Anti-inflammatory properties of bicyclic CB2 ligand in vitro.
[0358] The specific aspects of the inflammatory response cascade are achieved through cytokines such as tumor necrosis factor-α (TNF-α), IFN-γ, IL-2 and IL-1β, and such as COX-2 and PGE 2 Mediated by inflammatory mediators. Regulating the levels of these pro-inflammatory agents is very important for the severity of the final inflammatory outcome. These pro-inflammatory agents are also produced by activated cells of the immune system, and the purpose of this study is to test the effect of new bicyclic CB2 ligands on the secretion of these inflammatory agents from activated macrophages and T cells. The secretion level in various test groups was determined by ELISA analysis, and the inhibition level was calculated in comparison with the vehicle treatment group.
[0359] ELISA was used for protein quantification.
[0360] The quantification technique for a given protein in liquid samples, tissue culture supernatant...
Embodiment 3
[0378] The effect of the compound on gene expression.
[0379] The inhibitory activity shown by the effect of some bicyclic CB2 binding compounds on the secretion of inflammatory agents in cells activated by the immune system in vitro or in vivo may be related to the regulation of gene expression.
[0380] RNA preparation and real-time RT-PCR.
[0381] SV total RNA isolation system (Promega) was used to prepare total RNA. Cells or tissues are homogenized in lysis buffer. According to the instructions of the kit, the lysate was transferred to the RNA separation column, treated with DNAse, washed and eluted. The concentration of RNA was determined by GeneQuant II (Pharmacia-Amersham). Complementary DNA (cDNA) was synthesized from total RNA using SUPERSCRIPT II reverse transcriptase (LifeTechnologies). According to the kit instructions, combine 2μg of total RNA with oligonucleotides (dT) 15 The combination of primers, 0.5mM dNTP mix, 8 units of reverse transcriptase and other reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com