Industrial process for preparing beta-thymidine
A thymidine and industrial technology, applied in the field of industrial preparation of beta-thymidine, can solve the problems of difficult recovery, equipment corrosion, long route and the like, and achieve the effects of simple and easy production process, reduced production cost and shortened synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
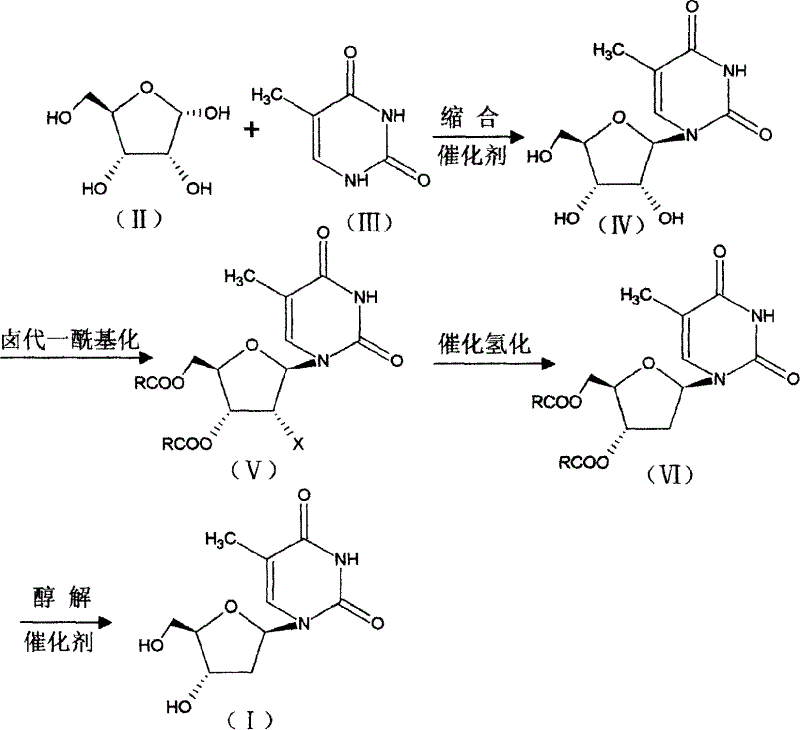
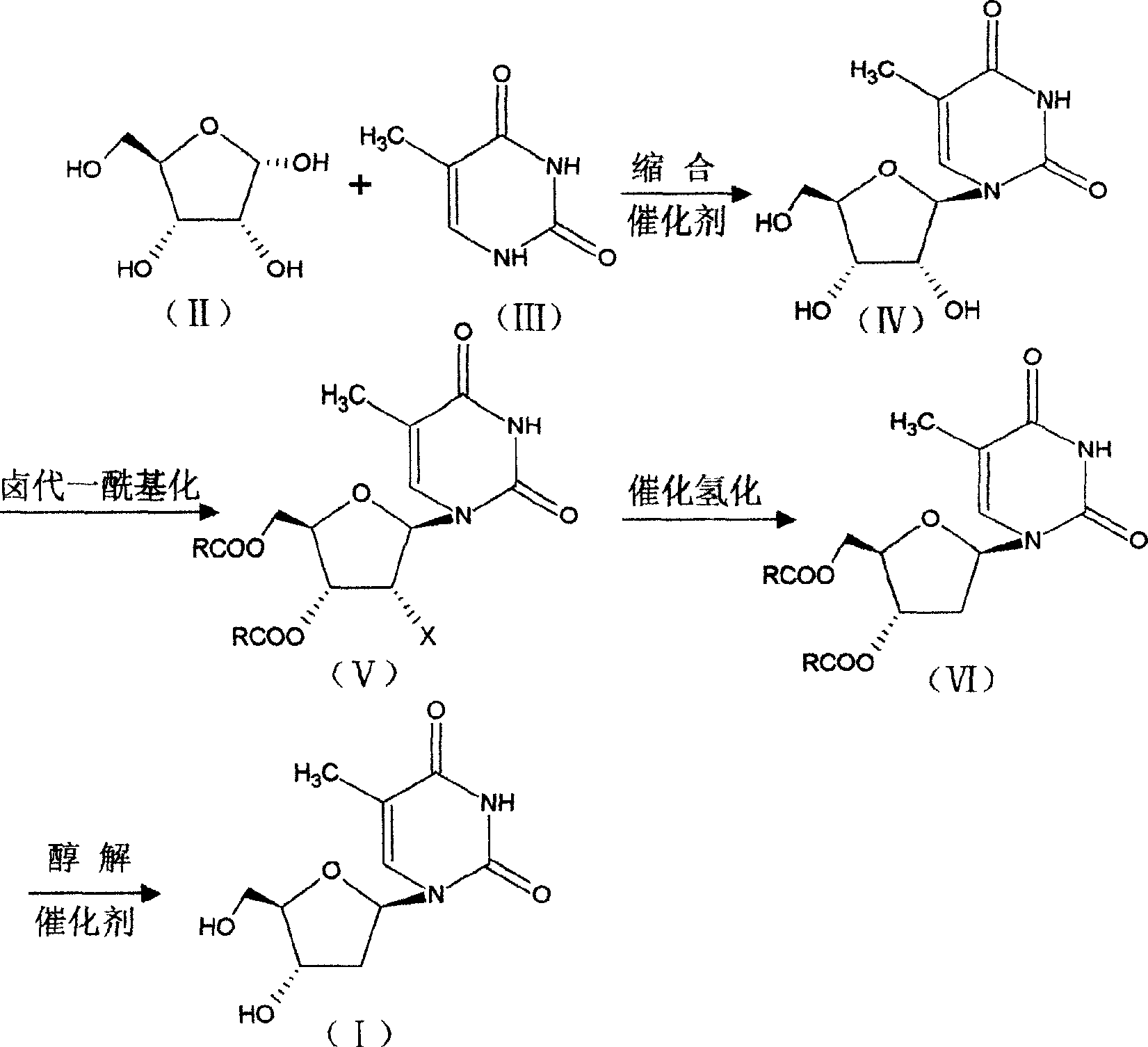
[0011] Add 15g (0.1mol) of D-ribose and 150ml of dichloromethane into a 250ml three-neck flask equipped with a stirrer, a thermometer, and a dropping funnel, stir until dissolved, then add 12.5g of thymine (0.1mol), and cool to 10 Below ℃, add 12ml of 98.5% concentrated sulfuric acid dropwise, after the addition is complete, naturally raise the temperature to 20-25℃, stir and react for 10 hours, add water, stir and separate layers, extract the aqueous layer with dichloromethane, combine the organic layers, and distill off dichloromethane Recrystallized from methane and ethanol to obtain 23 g of off-white 5-methyluridine, content (HPLC): 99.2%, melting point: 181-185°C: yield: 89.14%.
example 2
[0013] Add 150ml of acetonitrile and 30g (0.42mol) of propionyl chloride to a 500ml three-necked flask equipped with a stirrer and a thermometer, raise the temperature to 50°C, add 20g (0.077mol) of 5-methyluridine in batches, and keep stirring at 60°C for reaction For 3 hours, distill acetonitrile, cool, add 200ml of water, cool to below 5°C, keep stirring for 2 hours, filter with suction, wash with water, dry to obtain light yellow solid 2′-chloro-2′-deoxy-5-methyl-3 ', 5'-O-alkanoyl-β-D-riburidine 28g, melting point: 132-134°C, content (HPLC): 97.3%, yield: 93%.
example 3
[0015] Add 2'-chloro-2'-deoxy-5-methyl 4-3', 5'-O-alkanoyl-β-D-riburidine 39g (0.1mol) in the autoclave, add methanol / Water (1:0.3) 400ml, sodium acetate 12.5g (0.15mol), stir to dissolve, add Pd / C 40g, pass hydrogen to 3×10 5 Pa, react at 25--30°C for 4 hours, filter off the catalyst, concentrate under reduced pressure to obtain a viscous substance, which is directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com