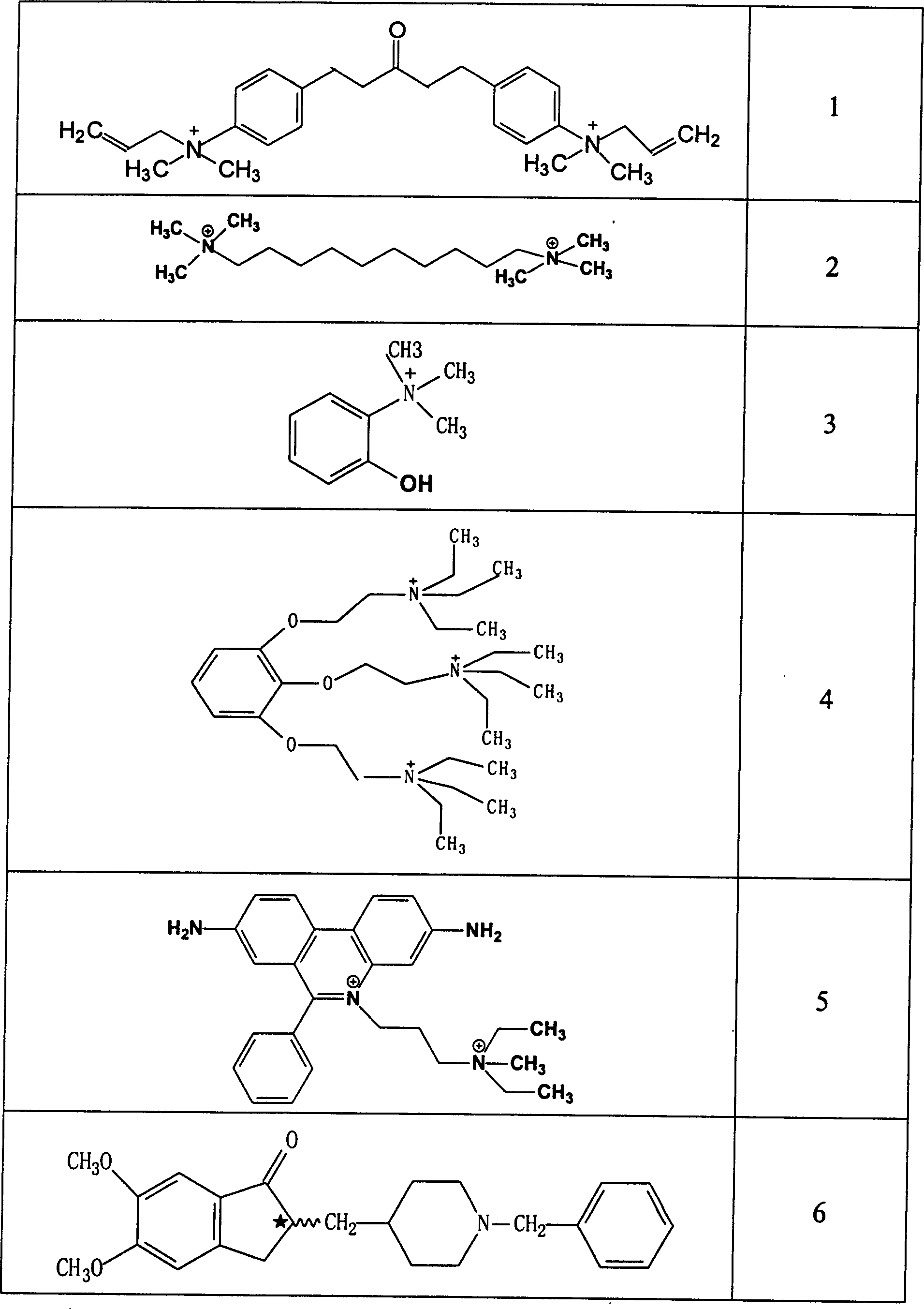
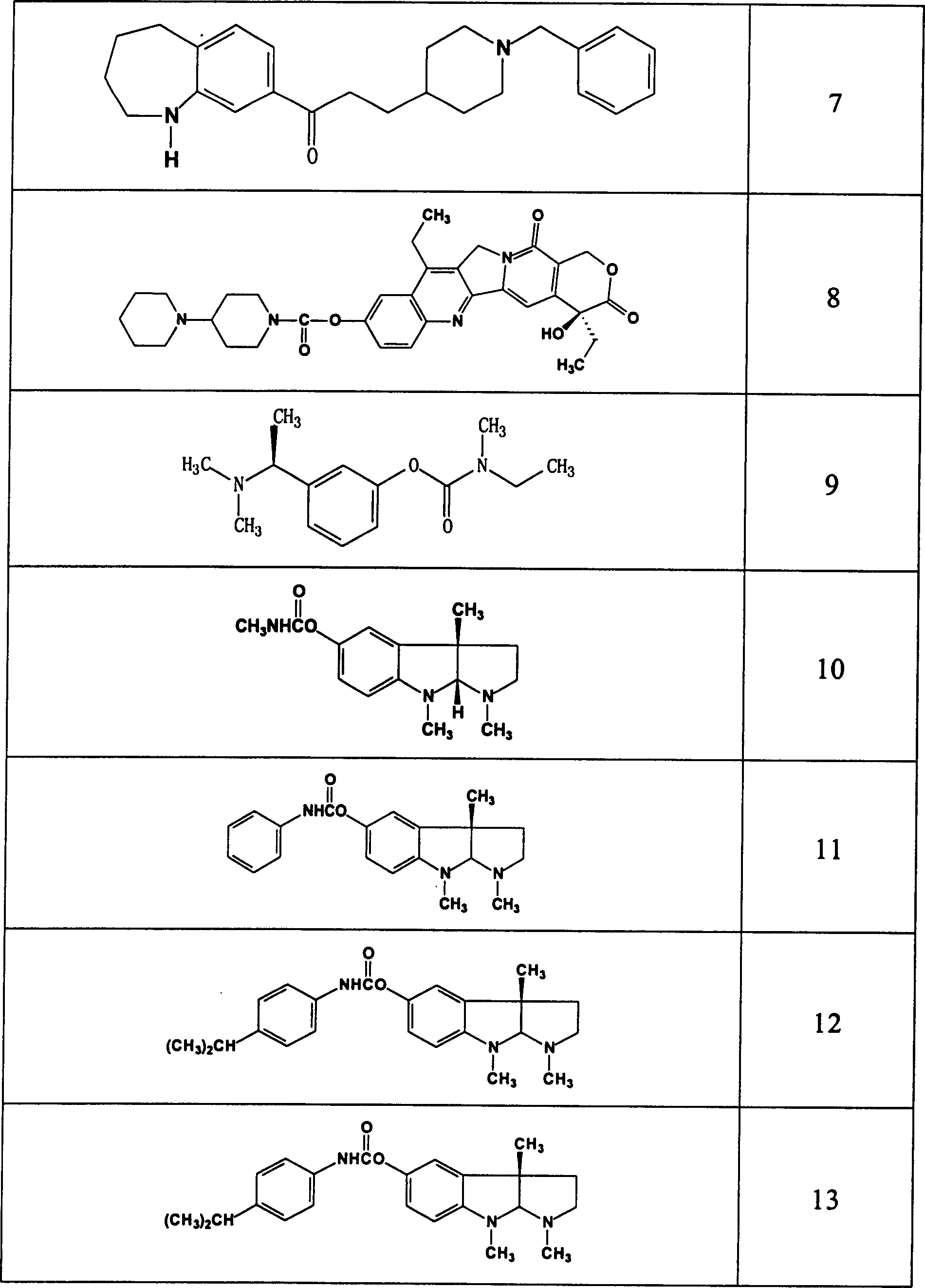
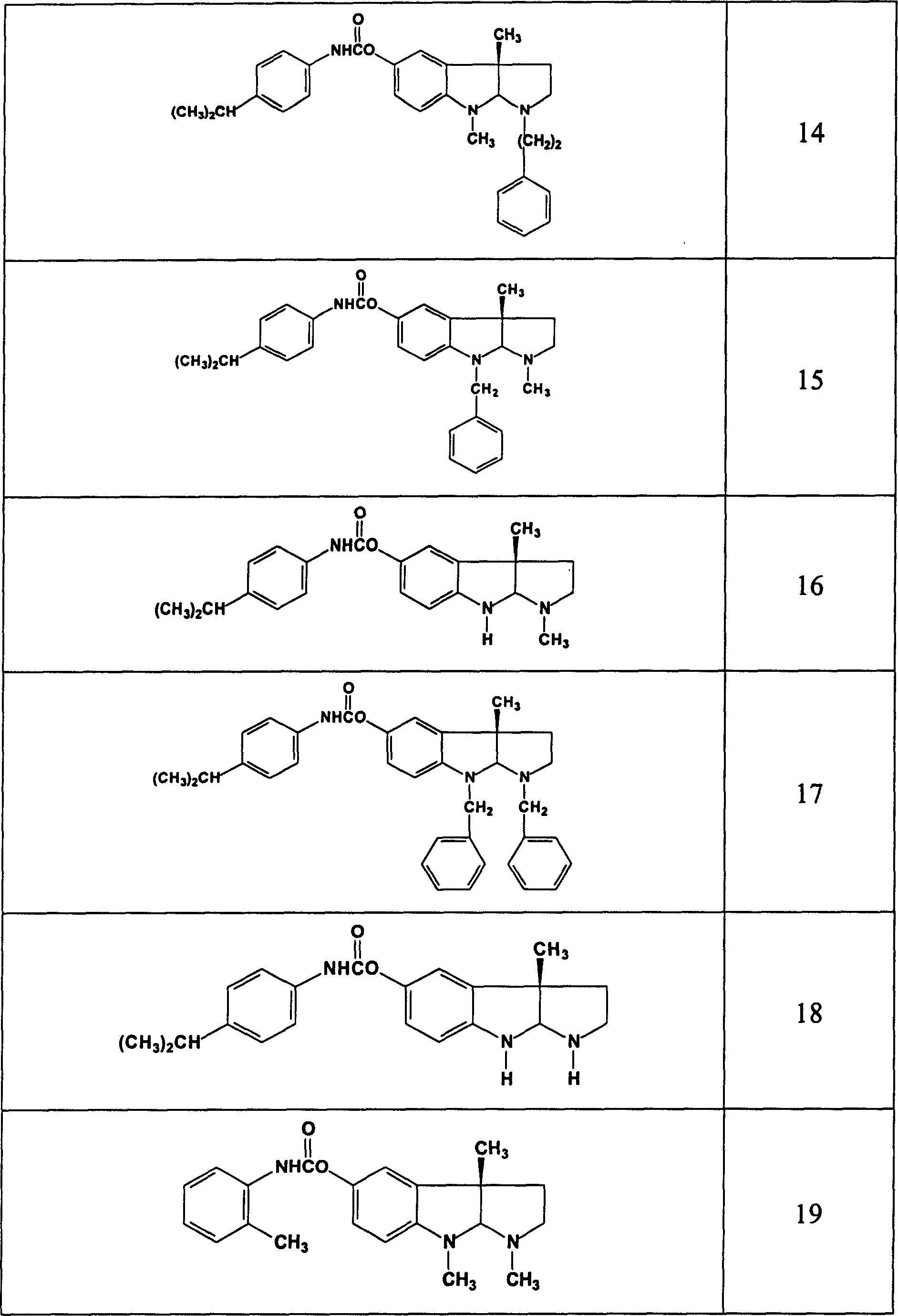
Use of acetylchloinesterase inhibitor for preparing medicine for treating parkinson's disease
A technology for acetylcholinesterase and Parkinson's disease, applied in the treatment of Parkinson's disease, in the field of acetylcholinesterase inhibitors, can solve the problems of inability to use and not very obvious effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Example 1 Detection of Acetylcholinesterase Expression in Apoptotic Cells in Vitro (Histochemical Method)
[0192] Cell culture: SK-N-SH cells, CHO cells: at 37.5°C, 5% CO 2 Under the moist conditions of the above, the cells were cultured in the RMBI-1640 (Gibco) medium of 10% calf serum (Huamei), adding HEPES and glutamine, 100IU / ml ampicillin, 100μg / ml streptomycin; the cells were treated with Subculture at a split ratio of 1:10 every week, culture for 8-12 days, pass for about 4-20 generations, and inoculate in 48-well culture plates (Costar) 2 days in advance until they are full and ready for use. A DAT-CHO cell line permanently expressing the rat dopamine transporter in a CHO cell line (designated D8 cell line). D8 was the same as CHO cell culture, except that Geneticin (G418) 400 μg / ml (Gibco / BRL) was added; SH-SY5Y cell culture used DMEM / F12 (Gibco) instead of RMBI-1640, and other conditions remained unchanged.
[0193] Induction of dopaminergic neurocytotoxici...
Embodiment 2
[0207] Example 2 Detecting the expression of acetylcholinesterase in dopaminergic neurotoxicity-induced cells from the mRNA level
[0208] The induction method is the same as above, collect the cells induced by dopamine and 6-hydroxydopamine, take the cells induced by serum culture as positive control and the uninduced normal cells as negative control; take 7×10 6 After the cells were lysed with 1ml Trizol, the total RNA was extracted with chloroform, isopropanol, and 75% ethanol; 2 μg of total RNA was taken to a 20 μl reaction system, and random primers were used to perform reverse transcription to generate cDNA; design upstream primers and Downstream primers: 5'-CGGGTCTACGCCTACGTCTTTGAACACCGTGCTTC-3' and 5'-CACAGGTCTGAGCAGCGATCCTGCTTGCTG-3'.
[0209] The product length is: 482bp. Use PE480 type PCR instrument, PCR reaction conditions: denaturation: 94°C, 60sec; annealing: 60°C, 70sec; extension 72°C, 90sec; 30 cycles; β-actin as internal reference.
[0210] result:
[021...
Embodiment 3
[0212] Example 3 MTT method to detect cell viability
[0213] SH-SY5Y cells were diluted to an appropriate concentration and inoculated in a 96-well plate in a volume of 200 μl. Induced treatment after 24 hours of culture. After dopamine induction, change the medium, add 20 μl (5 mg / ml) of MTT, 37 ° C for 4 hours, remove the medium, add 150 μl of dimethyl sulfoxide, shake and dissolve for 15 minutes, measure the O.D. value at 590 nm with a microplate reader, The group was repeated at least 3 times, and the average value was taken.
[0214] result:
[0215] Dopamine (200 μM) induces a large number of cell death, while the addition of huperzine A (1 μM) can partially prevent cell death, and there is a significant difference (p0.05), therefore, the acetylcholinesterase inhibitor in vitro could partially inhibit the cell death caused by dopaminergic neurotoxicity ( Figure 7 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com