Cephe alkene onium salt compound and its preparation and use in preparation of cefepime
A technology of cephems and compounds, applied in the field of preparing cefepime, can solve the problems of high cost, achieve the effects of reducing reaction steps, controlling environmental pollution, and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
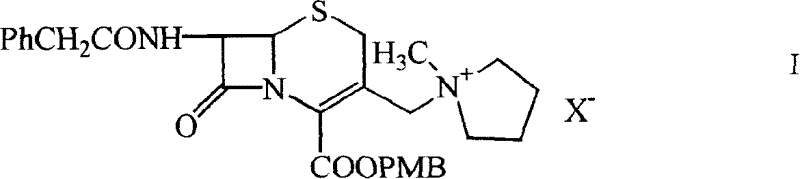
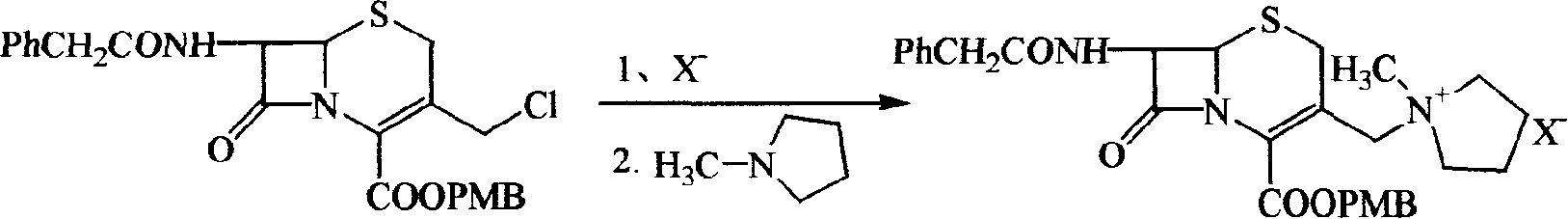
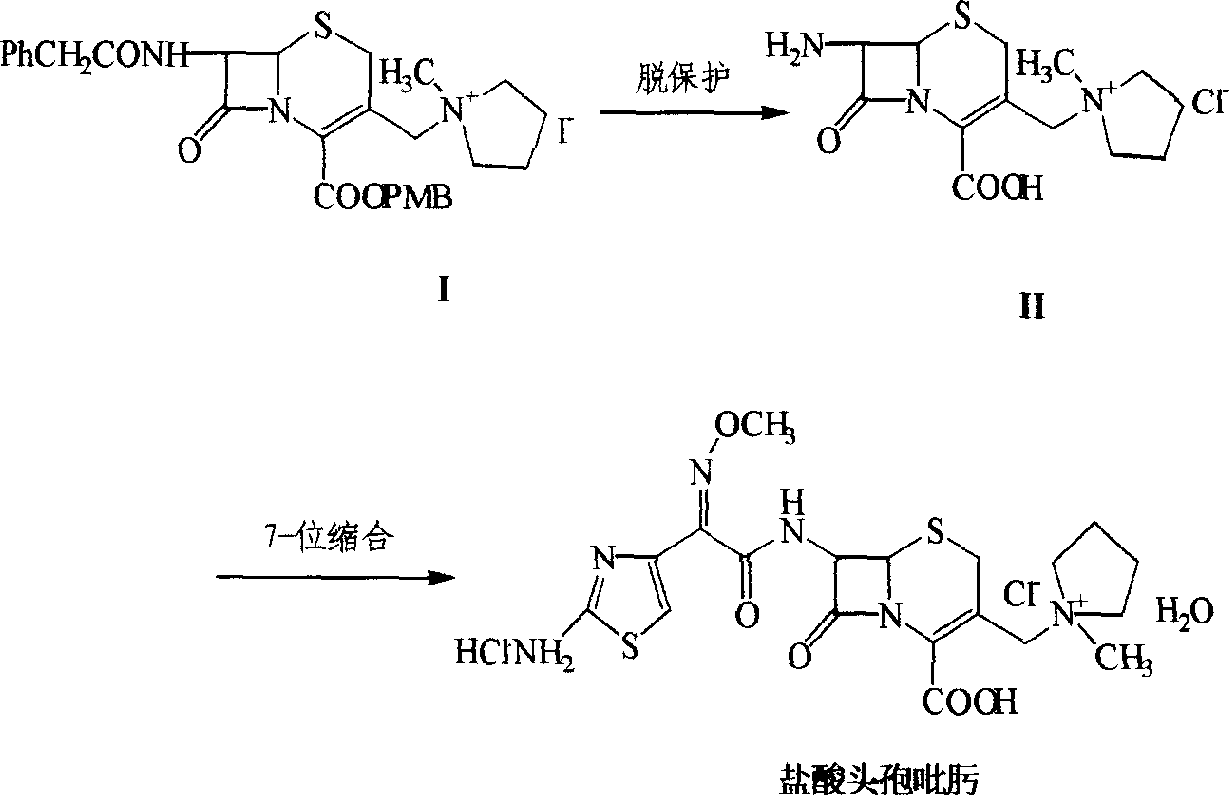
[0050] One, preparation 7-phenylacetamido-3-(1-methyl-1-pyrrolidinium base) methyl-3-cephem-4-carboxylate methoxybenzyl ester (I)
[0051] (A1) Preparation of iodide
[0052] Put 9.73g GCLE and 40ml dichloromethane into the reaction bottle, seal it and avoid light, stir in a water bath at 20-25°C, add dropwise a solution of 4.5g sodium iodide and 20ml acetone, react for 2 hours, drain the solvent, add 50ml dichloromethane, The reactant was dissolved, washed once with 100ml water, and then saturated with 50ml Na 2 S 2 o 3 Wash twice, add anhydrous Na 2 SO 4 Dry, filter, wash the filter cake with 10ml of dichloromethane, combine the filtrate into another dry reaction bottle, stir mechanically, cool down in an ice-salt bath, add 2.1ml of N-methylpyrrolidine dropwise when the internal temperature is ≤0°C, and react After 1 hour, the reaction solution was added into a stirred flask containing 600 ml of isopropanol, and a solid was precipitated, filtered with suction, washed tw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com