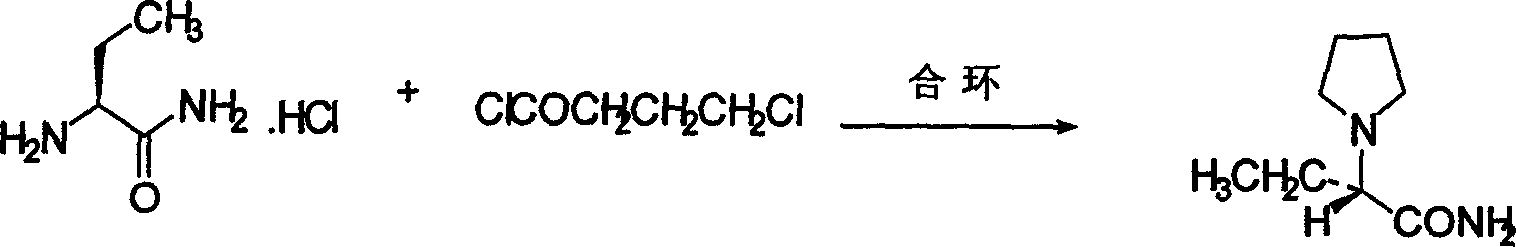Preparation method of (S)-alpha-ethyl-2-keto-1-pyrrolidine acetamide
A technology of pyrrolidine acetamide and pyrrole acetonitrile, which is applied in the field of new antiepileptic drugs, can solve the problems of unfavorable industrial production, high price of starting raw materials, and injury to operators, and achieve great implementation value and social and economic benefits, low cost, Effects of Effective Recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
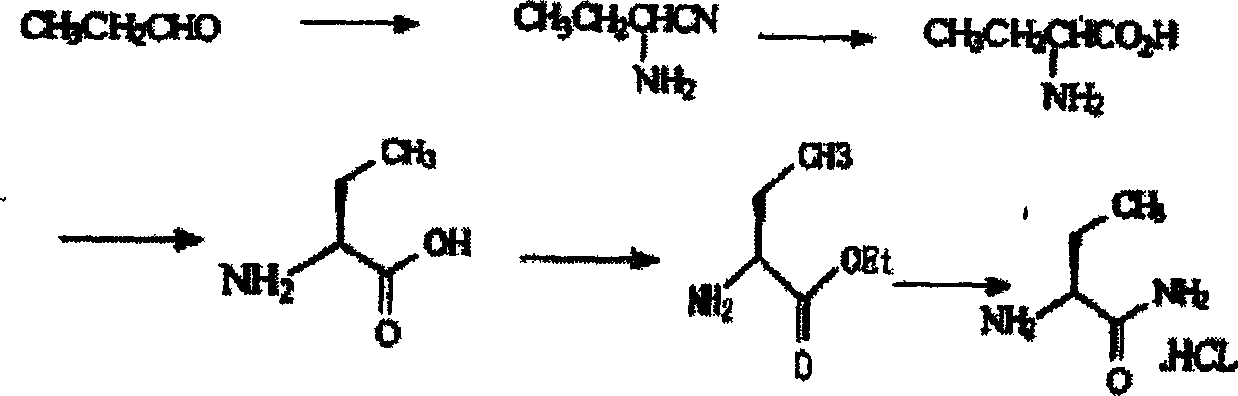
[0043] Embodiment 1: the synthesis of (S)-α-amino-butyrocyanine hydrochloride
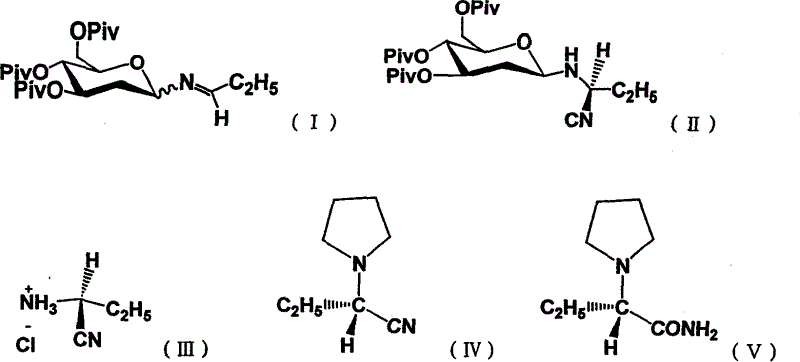
[0044] At -40°C to -20°C, 3.96 g of trimethylsilyl azide cyanide (40 mmol) and catalyst SnCl 4 (40mmol) in dichloromethane (400mL) solution was slowly added dropwise 20mL containing N-(3,4,6-tripivaloyl-2-deoxyglucosyl) propyl imine (30mmol) dichloromethane solution ; The catalyst here can also be ZnCl 2 or TiCl 4 After the dropwise addition, under stirring, the solution was slowly heated to -20°C, and the reaction was carried out at -20°C and monitored by TLC. After the reaction was completed, the solvent was evaporated, and then the remaining residue was dissolved in 400ml CH 2 Cl 2, the organic layer was sequentially washed with 2N HCL (100ml), saturated NaHCO 3 (3 × 200ml) and water (200ml) washing, organic layer with MgSO 4 Drying, after drying, the solvent is evaporated, then hydrolyzed in a saturated hydrogen chloride isopropanol solution to obtain a solid precipitate, and recrystallize...
Embodiment 2
[0045] Embodiment 2: the synthesis of (S)-α-amino-butyrocyanine hydrochloride
[0046] At -20°C to 0°C, 3.96 g of trimethylsilylazide cyanide (40 mmol) and catalyst SnCl 4 (40mmol) in dichloromethane (400mL) solution was slowly added dropwise 20mL containing N-(3,4,6-tripivaloyl-2-deoxyglucosyl) propyl imine (30mmol) dichloromethane solution ; The catalyst here can also be ZnCl 2 or TiCl 4 After the dropwise addition, under stirring, the solution was slowly heated to 0°C, and the reaction was carried out at 0°C and monitored by TLC. After the reaction was completed, the solvent was evaporated, and then the remaining residue was dissolved in 400ml CH 2 Cl 2 , the organic layer was sequentially washed with 2N HCL (100ml), saturated NaHCO 3 (3 × 200ml) and water (200ml) washing, organic layer with MgSO 4 After drying, the solvent was evaporated after drying, and then hydrolyzed in a saturated hydrogen chloride isopropanol solution to obtain a solid precipitate, which was rec...
Embodiment 3
[0047] Embodiment 3: the synthesis of (S)-α-amino-butyrocyanine hydrochloride
[0048] SnCl 4 The amount was changed to (60 mmol) and the others were the same as in Example 1 to obtain 3.2 g (S)-α-amino-butyrocyanine hydrochloride (yield 90.4%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com