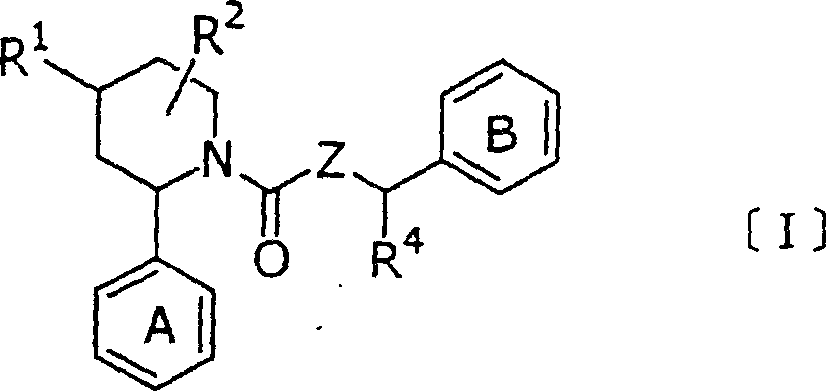
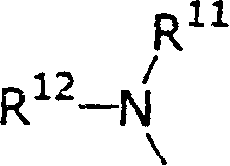
New piperidine derivative
A technology of piperidine compounds and compounds, applied in the direction of effective components of heterocyclic compounds, digestive system, organic chemistry, etc., can solve problems such as undiscovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0465](1) 1.43 g of 1-{N-(3,5-bistrifluoromethylbenzyl)-N-methyl}aminocarbonyl-2-(4-fluoro-2-methylphenyl) was dissolved in 30 ml of methanol )-4-oxopiperidine, to which 114 mg of sodium borohydride was added. The mixture was stirred at room temperature for 3 hours. To the reaction mixture were added an aqueous ammonium chloride solution and ethyl acetate, and after the mixture was stirred, the layers were separated. The organic layer was washed with water and brine, dried, and then the solvent was removed by distillation under reduced pressure. The residue was purified by silica gel column chromatography (chloroform:ethyl acetate=4:1) to give 0.99 g of 1-{N-(3,5-bistrifluoromethylbenzyl)-N-methyl}-aminocarbonyl -2-(4-Fluoro-2-methylphenyl)-4-hydroxypiperidine, as shown in Table 1 below.
[0466] (2) 200 mg of the compound of (1) above was further purified by silica gel column chromatography (hexane:ethyl acetate=4:1) to give 18 mg of (a) trans-1-{N-(3,5-bistris Fluorometh...
Embodiment 2
[0468] Dissolve 200 mg of (2R)-1-[N-{1-(S)-(3,5-bistrifluoromethylphenyl)ethyl}-N-methyl]aminocarbonyl-2-( in 10 ml of tetrahydrofuran 4-Fluoro-2-methylphenyl)-4-oxopiperidine, 60 mg of sodium borohydride was added thereto, and the mixture was refluxed. While the mixture was continuously refluxed, a mixed solvent of 1 ml of methanol and 5 ml of tetrahydrofuran was added dropwise thereto. After 5 hours, the reaction mixture was poured into water and the layers were separated. The aqueous layer was extracted with ethyl acetate, the combined organic layers were washed with water and saturated brine and dried, and then the solvent was removed by distillation under reduced pressure. The residue was purified by silica gel column chromatography (hexane:ethyl acetate=1:1-1:2) to provide 33 mg of (a)(2R,4R)-1-[N-{1-(S)-(3) , 5-bistrifluoromethylphenyl)ethyl}-N-methyl]aminocarbonyl-2-(4-fluoro-2-methylphenyl)-4-hydroxypiperidine and 160 mg (b)(2R , 4S)-1-[N-{1-(S)-(3,5-bistrifluorome...
Embodiment 3
[0470] (2R)-1-[N-(3,5-bistrifluoromethylbenzyl)-N-methyl]aminocarbonyl-2-(4-fluoro-2-methylphenyl)-4-oxo substituted piperidine, treated in the same manner as Example 2 to provide (a)(2R,4R)-1-{N-(3,5-bistrifluoromethylbenzyl)-N-methyl}aminocarbonyl -2-(4-Fluoro-2-methylphenyl)-4-hydroxypiperidine and (b)(2R,4S)-1-{N-(3,5-bistrifluoromethylbenzyl)- N-methyl}aminocarbonyl-2-(4-fluoro-2-methylphenyl)-4-hydroxypiperidine, as shown in Table 2 below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com