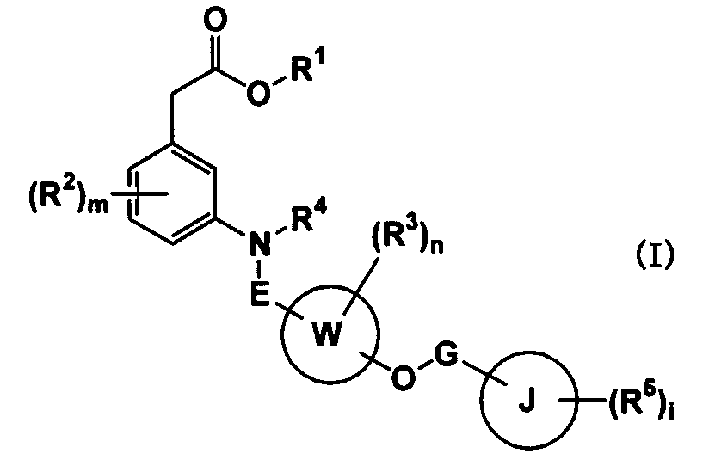
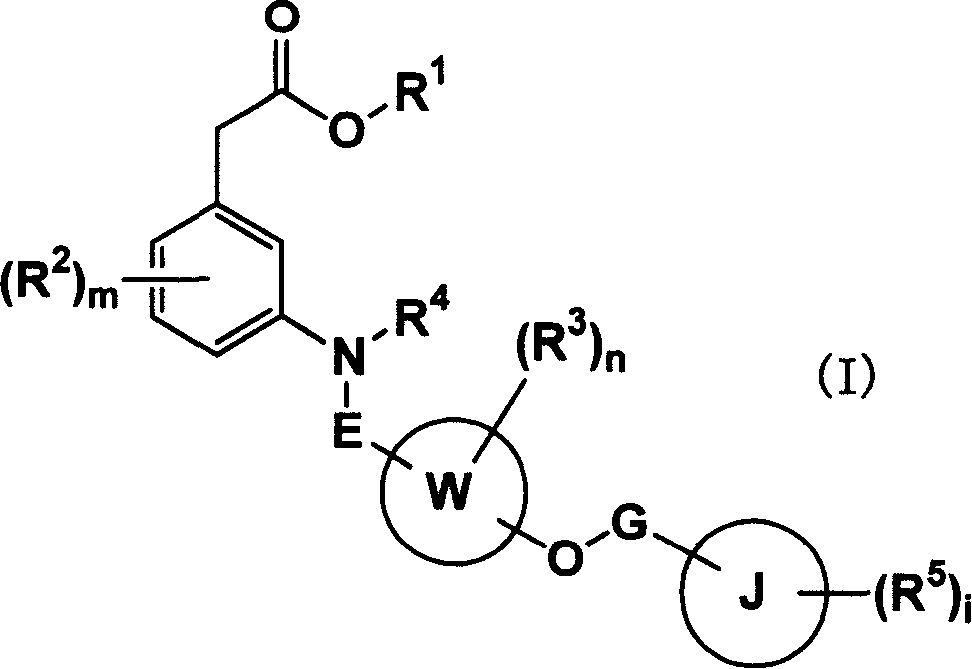
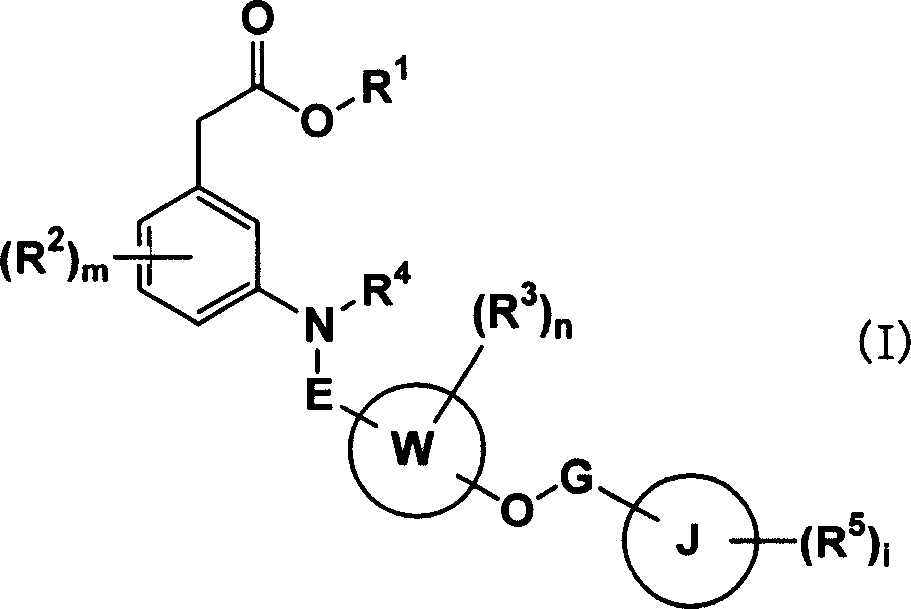
Carboxylic acid compounds and drugs containing the compounds as the active ingredient
A carboxylic acid compound and compound technology, applied in the direction of medical preparations containing active ingredients, organic active ingredients, drug combinations, etc., can solve the problems of small toxic and side effects, weak combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0195] [Preparation method of the compound of the present invention]
[0196] The compound of the present invention represented by formula (I) can be prepared by, for example, the following method:
[0197] [I] In the compound represented by formula (I), R 1 Is C 1-4 Alkyl, C 2-4 Alkenyl or benzyl compounds are compounds represented by the following formula (IA)
[0198]
[0199] (In the formula, R 1A Is C 1-4 Alkyl, C 2-4 (Alkenyl or benzyl, other symbols have the same meaning as above) can be prepared by the following method:
[0200] (a) In formula (IA), E represents -C(=O)- or -S(O) 2 -The compound is the compound of formula (IA-1)
[0201]
[0202] (In the formula, E A Is -C(=O)- or -S(O) 2 -, the meaning of other symbols is the same as above) can be prepared by the following method: compound of formula (II-1)
[0203]
[0204] (In the formula, R 2-1 With R 2 Has the same meaning, but R 2-1 The indicated hydroxyl or amino group is protected when necessary; R 4-1 Is a hydr...
Embodiment approach
[0343] The following reference examples and examples are used to illustrate the present invention, but cannot be used to limit the present invention.
[0344]In chromatographic separation or TLC, the solvent in parentheses represents the eluent or developing agent, and the solvent ratio used is the volume ratio. In NMR, the solvent in parentheses is the solvent for measurement.
[0345] Reference Example 1: N-formyl-2-fluoroaniline
[0346] In an argon atmosphere at 0°C, formic acid (6.1 mL) was added dropwise to acetic anhydride (15.5 mL), and the mixture was stirred at 50°C for 2 hours. After the reaction mixture was cooled to room temperature, it was diluted with tetrahydrofuran (THF; 10 mL). To the above diluted solution was added a solution of 2-fluoroaniline (5.56 g) in THF (20 mL) at room temperature, and then the mixture was stirred at room temperature for 1 hour. The reaction mixture was concentrated to obtain the title compound with the following physical and chemical da...
Embodiment 1
[0384] Example 1: 3-(4-((2S)-4-methyl-3,4-dihydro-2H-1,4-benzoxazin-2-ylmethoxy)benzoylamino)benzene Methyl acetate
[0385]
[0386]Under the protection of argon, pyridine (161 μl) was added to the dichloromethane (2 mL) solution of the compound (165 mg) prepared in Reference Example 9. Under ice cooling, a dichloromethane (2.5 mL) solution of the compound (350 mg) prepared in Reference Example 8 was added dropwise to the mixture, and the mixture was stirred at 0°C for 15 minutes. Methanol and water are added to this mixture. It was extracted with ethyl acetate, and the organic layer was washed with saturated aqueous ammonium chloride solution and saturated sodium chloride aqueous solution, dried over anhydrous sodium sulfate, and concentrated to obtain the title compound (447 mg) having the following physical data.
[0387] TLC: Rf 0.23 (hexane:ethyl acetate=2:1).
[0388] Examples 1(1)~1(15)
[0389] In the same manner as in Example 1, using the corresponding amine instead of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com