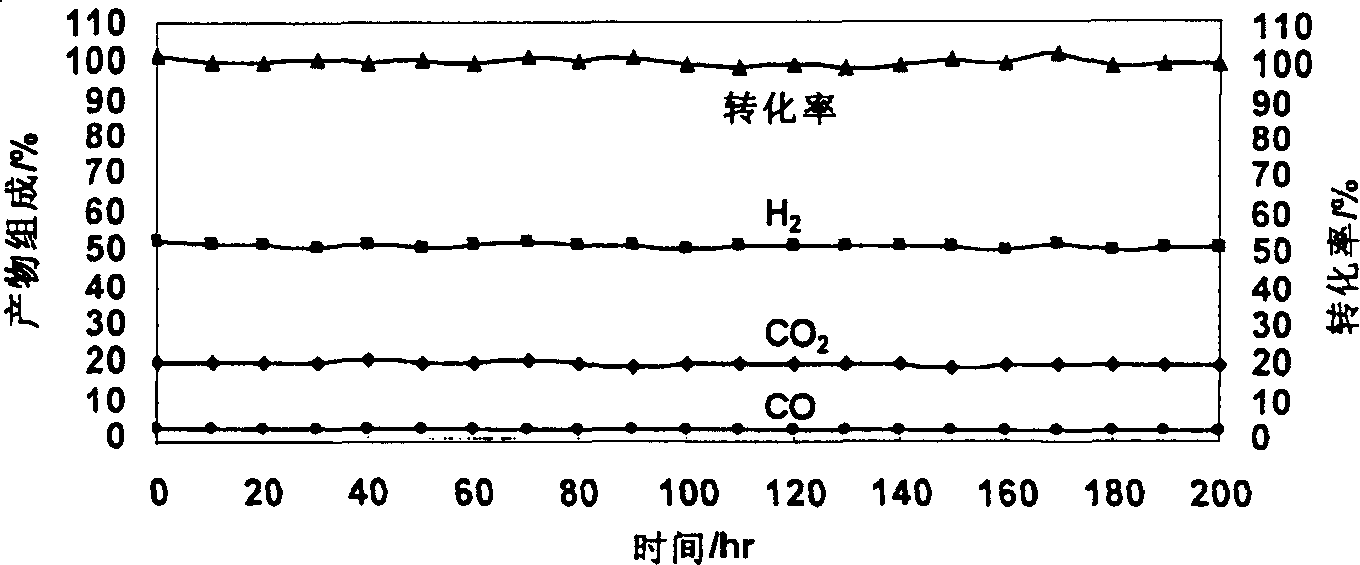
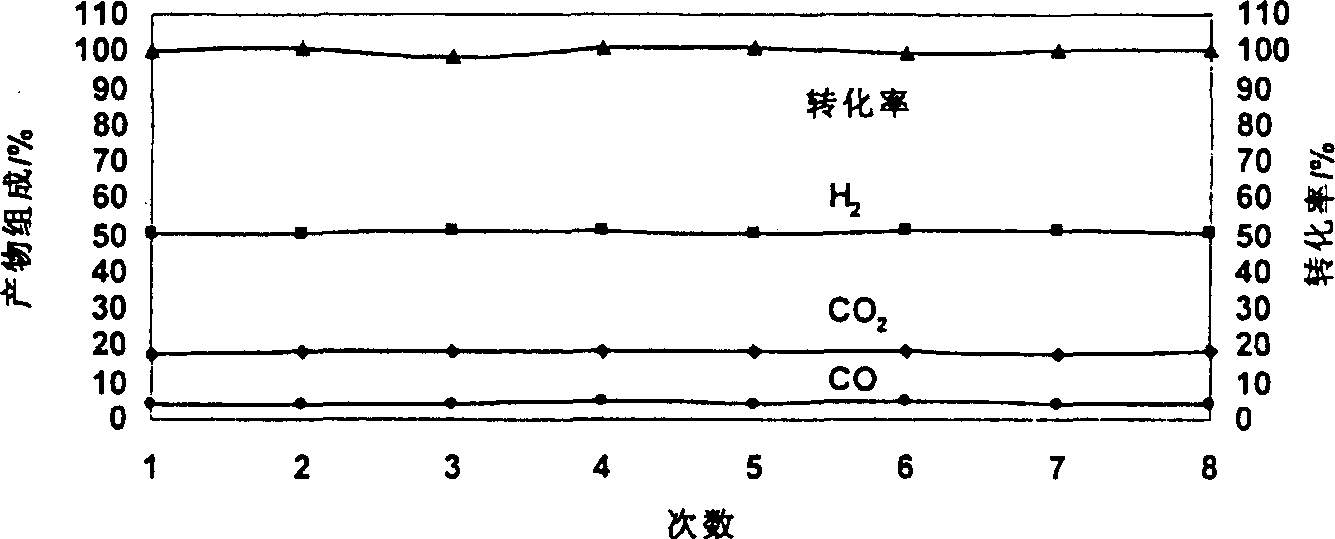
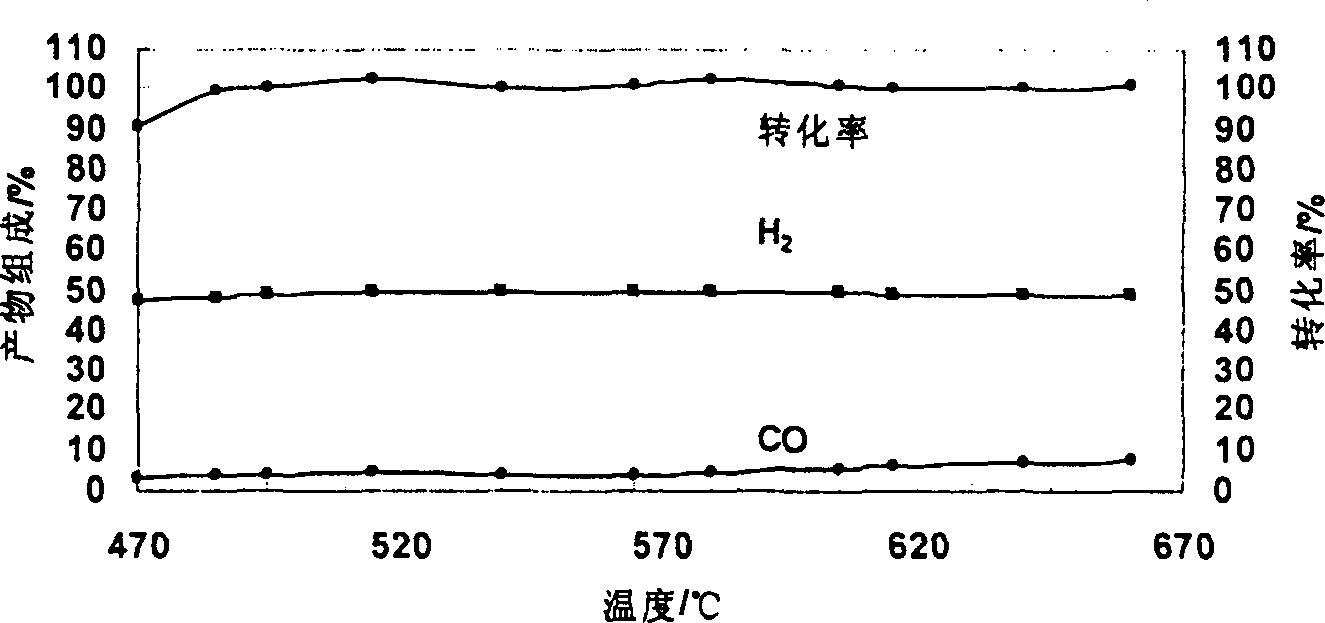
Catalyst for autothermal reformation of methanol to prepared hydrogen and its prepn process and application
A technology of autothermal reforming and catalysts, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc. Expensive and other issues, to achieve good heat stability, high activity, good strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] Example 1: ZnO-Cr prepared by coprecipitation method 2 o 3 -CeO 2 -La 2 o 3 -ZrO 2 composite oxide catalyst
[0033] a) Weigh industrial grade Ce(NO 3 ) 3 ·6H 2 O 90.90g, technical grade Zr(OH) 4 8.45g, La(NO 3 ) 3 ·6H 2 O6.35g. Will weigh Zr(OH) 4 Put it into a beaker, add 65-68% concentrated nitric acid and heat to react until there are no visible particles and the solution is transparent. The dissolved Zr(NO 3 ) 4 The solution was poured into the dissolved Ce(NO 3 ) 3 and La(NO 3 ) 3 The mixed solution was filtered for later use.
[0034] b) Weighing analytically pure Zn(NO 3 ) 2 ·6H 2 O 18.90g, (NH 4 ) 2 Cr 2 o 7 5.55g, add deionized water to dissolve, then mix the Ce-Zr solution in step a with it, and in the case of constant stirring, use a separatory funnel to drop 25-28% ammonia water into the above mixed solution, the amount of ammonia water depends on the pH Control the value until the pH value reaches 7-8. The formed Zn-Cr-Ce-Zr c...
example 2
[0035] Example 2: ZnO-Cr prepared by impregnation method 2 o 3 / CeO 2 composite oxide catalyst
[0036] a) Weigh industrial grade Ce(NO 3 ) 3 ·6H 2 O 90.90g, add deionized water to dissolve, filter, drop 25-28% ammonia water into the above solution with a separatory funnel, the amount of ammonia water is controlled according to the pH value, until the pH value reaches 8-9. Formed CeO 2 The precipitate was thoroughly stirred, vacuum filtered and washed, and then put into an oven for drying at 110°C for 15 hours, and then put into a muffle furnace for calcination at 500°C for 2 hours. Grind the roasted product to below 200 mesh, add 1.5g of pseudo-boehmite and 1.5ml of 10% nitric acid, mix well, extrude with extruder, air-dry and cut into cylinders about 3×4mm. Dry the cylinder in an oven at 110°C for 4 hours, then put it in a muffle furnace and bake it at 500°C for 2 hours to obtain CeO 2 carrier. Grind it to 12-16 mesh and measure its water absorption.
[0037] b) W...
example 3
[0038] Example 3: ZnO-Cr prepared by thermal decomposition 2 o 3 -CeO 2 -ZrO 2 composite oxide catalyst
[0039] a) Weigh industrial grade Ce(NO 3 ) 3 ·6H 2 O 90.90g, technical grade Zr(OH) 4 8.45g, analytically pure Zn(NO 3 ) 2 ·6H 2 O 18.90g, (MH 4 ) 2 Cr 2 o 7 5.55g, urea 0.5g. Mix the above reagents well, put them into a muffle furnace, conduct thermal decomposition at 500°C for 30 minutes, and cool down to room temperature rapidly after the decomposition is completed.
[0040] b) Grind the decomposition product to below 200 mesh, add 2.5g of pseudo-boehmite, mix well, extrude with extruder, air-dry and cut into cylinders of about 3×4mm. Dry the cylinder at 110°C for 4 hours in an oven, and then bake it in a muffle furnace at 800°C for 2 hours to obtain ZnO-Cr 2 o 3 -CeO 2 -ZrO 2 Composite oxide catalyst (C).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com