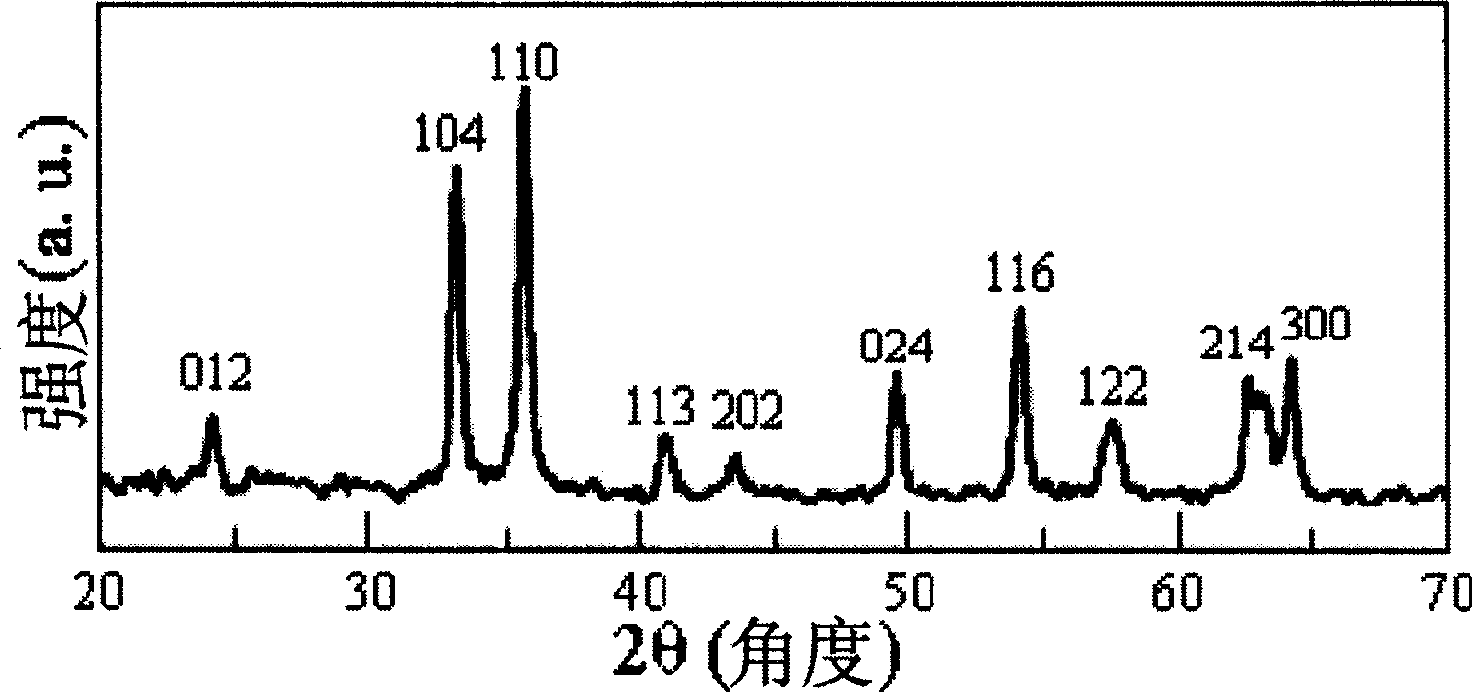
Catalyst irontrioxide for carbon monoxide oxidation reaction and its preparing method
An oxidation reaction, carbon monoxide technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of poor catalyst recycling performance, high cost, synthesis Complicated technology and other issues, to achieve good recycling performance, low cost, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 2.5 mmol ferrous chloride (FeCl 2 ) and ligand 7.5mmol o-phenanthroline (1,10-phenanthroline), then add 50ml of water, and keep the temperature at 10°C for 24 hours; the filtered product is washed twice with water, placed in a vacuum oven and dried at 60°C for 3 hours. 0.05 g of intermediate product β-FeOOH powder was obtained. The aqueous solution that stays after filtering, according to the weight 0.05g (0.56mmol) of gained product, calculates that remaining iron protochloride content is 1.94mmol in the solution, then replenishes 0.56mmol ferrous chloride in the aqueous solution that stays after filtering Iron, continue to react again, let the utilization rate of o-phenanthroline reach 100%. Heat the obtained β-FeOOH powder to 520°C in a vacuum environment and keep it for 16 hours to obtain α-Fe 2 o 3 Products, the vacuum degree is 99.99%.
Embodiment 2
[0020] Add 2.5 mmol ferrous chloride (FeCl 2 ) and ligand 7.5mmol o-phenanthroline (1,10-phenanthroline), then add 50ml of water, and keep the temperature at 15°C for 18 hours; the filtered product is washed twice with water, placed in a vacuum drying oven at 40°C and dried for 5 hours. 0.05 g of intermediate product β-FeOOH powder was obtained. The aqueous solution that stays after filtering, according to the weight 0.05g (0.56mmol) of gained product, calculates that remaining iron protochloride content is 1.94mmol in the solution, then replenishes 0.56mmol ferrous chloride in the aqueous solution that stays after filtering Iron continues to react, allowing the utilization rate of o-phenanthroline to reach 100%. Heat the obtained β-FeOOH powder to 530°C in a vacuum environment for 14 hours to obtain α-Fe 2 o 3 The product, the vacuum degree is 99.99%.
Embodiment 3
[0022] Add 2.5 mmol ferrous chloride (FeCl 2 ) and ligand 7.5mmol o-phenanthroline (1,10-phenanthroline), then add 50ml of water, and keep the temperature at 25°C for 12 hours; the filtered product is washed twice with water, placed in a vacuum drying oven at 80°C and dried for 1 hour. 0.05 g of intermediate product β-FeOOH powder was obtained. The aqueous solution that stays after filtering, utilizes the remaining ferrous chloride content in the detection solution of fluorometer to also be 1.94mmol, then replenishes 0.56mmol ferrous chloride in the aqueous solution that stays after filtering, continues reaction, allows o-dinitrogen The utilization rate of Fei reached 100%. Heat the obtained β-FeOOH powder to 540°C in a vacuum environment for 12 hours to obtain α-Fe 2 o3 The product, the vacuum degree is 99.99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com