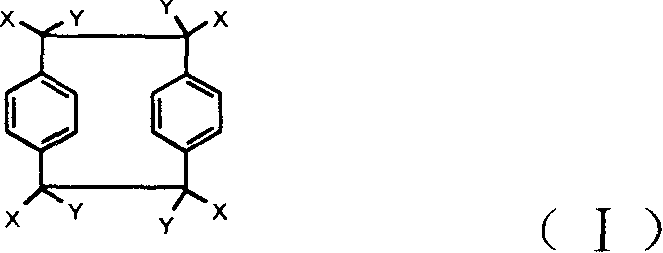Method for synthesizing fluoro-polypxylene
A technology of parylene and p-xylene, applied in chemical instruments and methods, halogenated hydrocarbon preparation, organic chemistry and other directions, can solve problems such as difficulty in realizing industrialization, no literature reports, etc., and achieve stable yield, short synthesis route, Effects suitable for mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
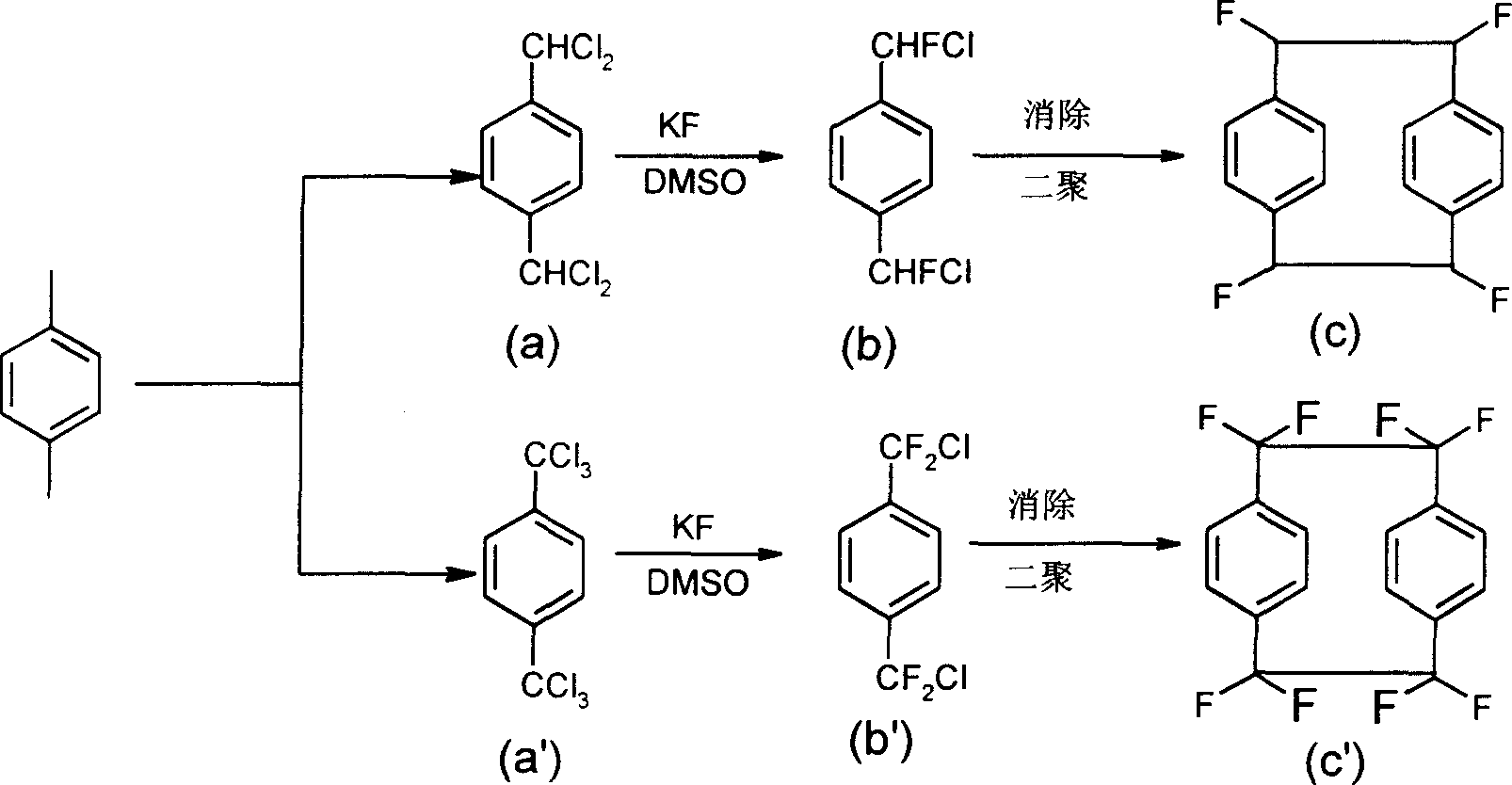
[0021] Add 48.4g (0.2mol) of raw materials p-dichlorotoluene and 200ml dimethyl sulfoxide into a 500mL three-necked flask, add 29g (0.5mol) of potassium fluoride, then add catalyst tetrabutylammonium chloride 0.01mol, heat to 140 ℃ reaction for 6 hours, after the reaction is completed, slowly add 100ml of water and 100ml of toluene after cooling down to room temperature, continue to stir for 30 minutes, then separate the reaction mixture, separate the organic phase, and extract the aqueous phase twice with 100ml of toluene*2, Combine the organic phases, wash the organic phase once with saturated sodium bicarbonate solution and saturated brine, dry over anhydrous sodium sulfate, remove toluene by rotary evaporation, and distill under reduced pressure to obtain the product p-chlorofluorotoluene.
[0022] Add Pd / C / Al8.0g (5% Pd / C6g mixed with 2g Al powder) into a 500mL three-necked flask, then add 200mL of toluene, stir for 5 minutes, cool to -5°C in an ice-water bath, and then ad...
Embodiment 2
[0024] Add 62g (0.2mol) of α-perhalogenated p-xylene and 200ml dimethyl sulfoxide into a 500mL three-necked flask, add 52.2g (0.9mol) of potassium fluoride, then add a catalytic amount of tetrabutylammonium chloride, heat React at 150°C for 8 hours. After the reaction is finished, after cooling down to room temperature, slowly add 100ml of water and 100ml of toluene, continue to stir for 30 minutes, then separate the reaction mixture, separate the organic phase, and extract the aqueous phase twice with 100ml of toluene*2, combine the organic phase, organic The phase was washed once with saturated sodium bicarbonate solution and saturated brine, dried over anhydrous sodium sulfate, toluene was removed by rotary evaporation, and the product p-difluoro-chlorotoluene was obtained by distillation under reduced pressure.
[0025] Add Pd / C / Al8.0g (5% Pd / C6g mixed with 2g Al powder) into a 500mL three-necked flask, then add 200mL of toluene, stir for 5 minutes, cool to -5°C in an ice-...
Embodiment 3
[0027] Add 48.4g (0.2mol) of raw materials p-dichlorotoluene and 200ml dimethyl sulfoxide into a 500mL three-necked flask, add 40g (0.7mol) of potassium fluoride, then add 2.5g of catalyst tetrabutylammonium chloride, and heat to 170 ℃ reaction for 4 hours, after the reaction is completed, slowly add 100ml of water and 100ml of toluene after cooling down to room temperature, continue to stir for 30 minutes, then separate the reaction mixture, separate the organic phase, and extract the aqueous phase twice with 100ml of toluene*2, Combine the organic phases, wash the organic phase once with saturated sodium bicarbonate solution and saturated brine, dry over anhydrous sodium sulfate, remove toluene by rotary evaporation, and distill under reduced pressure to obtain the product p-chlorofluorotoluene.
[0028] Add 8.0g Ni / C / Zn (5% Ni / C6g mixed with 2g Zn powder) into a 500mL three-necked flask, then add 200mL dimethyl sulfoxide, stir for 5 minutes, cool to -5°C in an ice-water bath...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com