New method for synthesizing Ramosetron Hydrochloride
A technology of ramosetron hydrochloride and a new method, applied in the field of preparation of tetrahydrobenzimidazole derivatives, can solve problems such as unfavorable industrial production, difficult separation, purification, low yield and the like, and achieve high product yield and high quality Controllable and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Preparation of 5-carboxylate methyl benzimidazole sulfate
[0026] Add 3200ml of methanol and 304g of 5-carboxylate benzimidazole sulfate in a 5L reaction flask, add 352ml of concentrated sulfuric acid dropwise under stirring, heat and reflux for 7 hours, cool, add 20g of activated carbon, heat and reflux for 0.5 hours, heat Filtrate, concentrate the filtrate, cool to obtain white crystals, filter and dry to obtain 240g, melting point: 169-171°C, yield 74%.
[0027] Reference Example 2
[0028] Preparation of 4,5,6,7-tetrahydrobenzimidazole-5-carboxylic acid sulfate (II)
[0029] In a 2L autoclave, add 1200ml of acetic acid, 80g of 5-carboxylate benzimidazole methyl sulfate, 40g of 10% Pd / C (dry weight), 80°C, 60kg / cm 2 Hydrogenation reaction under certain pressure conditions for about 16 hours (until the pressure no longer changes), cooling, discharging, filtering the catalyst, and concentrating under reduced pressure to obtain a light yellow oil, adding 300ml of 6N ...
Embodiment 1
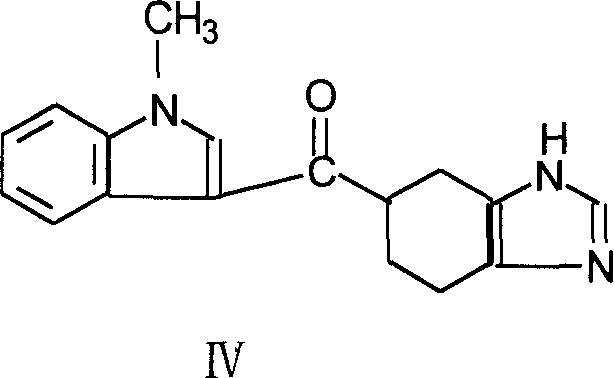
[0031] Preparation of 1-acetyl-5-carboxylic acid-4,5,6,7-benzimidazole (III)
[0032] Add 66g of 4,5,6,7-tetrahydrobenzimidazole-5-carboxylic acid sulfate, 62.0g of triethylamine, and 300ml of acetonitrile into the reaction flask, add dropwise 36.2g of acetyl chloride, heat and reflux and stir for 4 Hours, the acetonitrile layer was concentrated to dryness under reduced pressure, cooled, 300ml of water was added dropwise, extracted with ethyl acetate, dried, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 70 g of brown oil, which could be directly put into the next step for reaction.
Embodiment 2
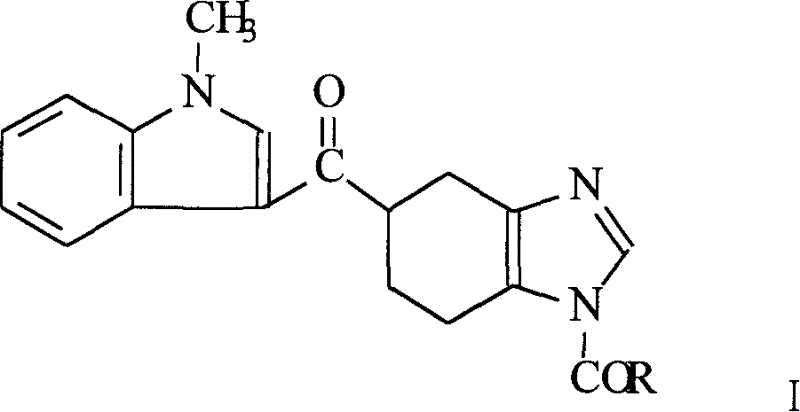
[0034] Preparation of 1-acetyl-5-[(1-methylindol-3-yl)carbonyl]-4,5,6,7-tetrahydrobenzimidazole (I)
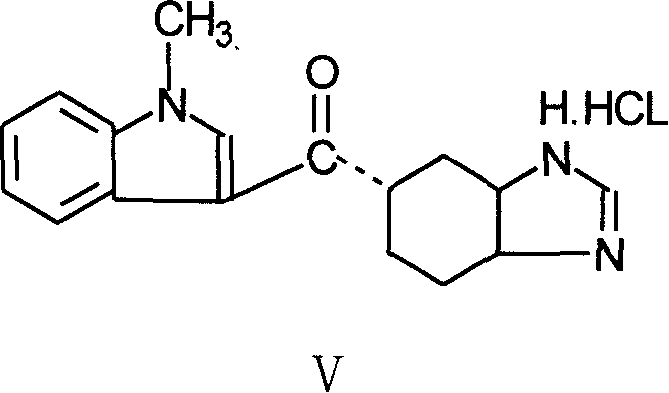
[0035] Add 70 g of acetyltetrahydrobenzimidazole, 200 ml of acetonitrile, and 40 ml of thionyl chloride into the reaction flask, heat to reflux, stir for 2 hours, and distill off part of the solvent under reduced pressure to take out excess thionyl chloride. After cooling, slowly add 53 g of tetrahydropyrrole and 100 ml of acetonitrile solution dropwise at 2°C. After the drop is complete, warm up to room temperature, stir for 2 hours, then 1 hour at 40°C, and concentrate under reduced pressure to obtain 132 g of a brown oily substance.
[0036] Add 132g of brown oil and 100g of N-methylindole to 300ml of acetonitrile, add 132g of phosphorus oxychloride, stir for 7 hours under heating and vigorous reflux, and cool to room temperature. Under cooling conditions, slowly add 660ml of water dropwise, stir with 500ml of ethyl acetate, separate layers, wash the organic layer twice wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com