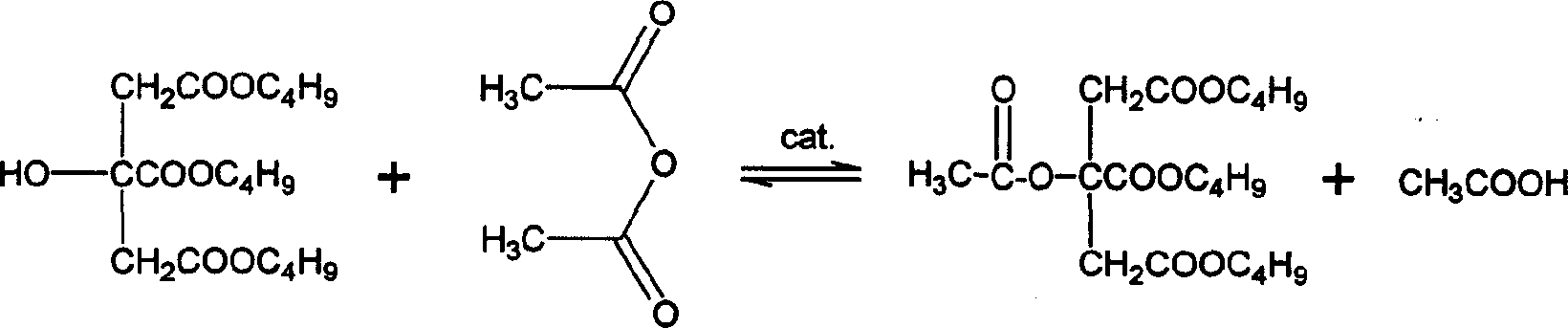
Catylatic synthesizing method of acetyl tri-in-butyl citrate
A technology for the synthesis of acetyl tri-n-butyl citrate, which is applied in chemical recovery, organic chemistry, etc., can solve the problems of complex product refining, many side reactions, and high price, achieve excellent catalytic performance, avoid separation difficulties, and prepare simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] According to the molar ratio of tri-n-butyl citrate and acetic anhydride as 1:1.8, weigh 18.02 g of tri-n-butyl citrate, 9.18 g of acetic anhydride, and 0.54 g of catalyst sodium bisulfate, put them into a three-necked flask, and place them in a water bath. Keep the temperature of the reaction solution at 60°C, stir and react for 90 minutes, and the yield of acetyl tributyl citrate is 99.3%. There is no peak of tri-n-butyl citrate on the gas chromatogram, indicating that tri-n-butyl citrate has been completely converted . After the crude product obtained by the reaction is suction filtered to recover the catalyst, acetic anhydride and acetic acid are recovered by distillation under reduced pressure, and then washed with deionized water, 5% sodium carbonate aqueous solution, and then washed with water until the water phase is neutral, and the water is removed by distillation under reduced pressure , That is, tri-n-butyl acetyl citrate. According to gas chromatography an...
Embodiment 2
[0018] According to the molar ratio of tri-n-butyl citrate and acetic anhydride as 1:1.5, weigh 18.02 g of tri-n-butyl citrate, 7.65 g of acetic anhydride, and 0.90 g of catalyst potassium bisulfate, put them into a three-necked flask, and place them in a water bath. Keep the temperature of the reaction solution at 70°C, stir and react for 60min, the yield of acetyl tri-n-butyl citrate is 99.5%, and there is no peak of tri-n-butyl citrate on the gas chromatogram, indicating that tri-n-butyl citrate has been Totally converted. After the crude product obtained by the reaction is suction filtered to recover the catalyst, acetic anhydride and acetic acid are recovered by distillation under reduced pressure, and then washed with deionized water, 10% aqueous sodium hydroxide solution, and then washed with water until the water phase is neutral, and then removed by distillation under reduced pressure. water, that is, acetyl tri-n-butyl citrate. According to gas chromatography analys...
Embodiment 3
[0020] According to the molar ratio of tri-n-butyl citrate and acetic anhydride as 1:1.8, weigh 18.02 g of tri-n-butyl citrate, 9.18 g of acetic anhydride, and 0.54 g of catalyst H-type macroporous strong acid cation exchange resin, and add them to a three-necked flask , placed in a water bath, keeping the temperature of the reaction solution at 50°C, stirring and reacting for 45 minutes, the yield of acetyl tri-n-butyl citrate was 99.4%, and there was no peak of tri-n-butyl citrate on the gas chromatogram, indicating that citric acid Tri-n-butyl ester has been completely converted. After the crude product obtained by the reaction is suction filtered to recover the catalyst, acetic anhydride and acetic acid are recovered by distillation under reduced pressure, and then washed with deionized water, 10% aqueous sodium bicarbonate solution, and then washed with water until the water phase is neutral, and then removed by distillation under reduced pressure. water, that is, acetyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com