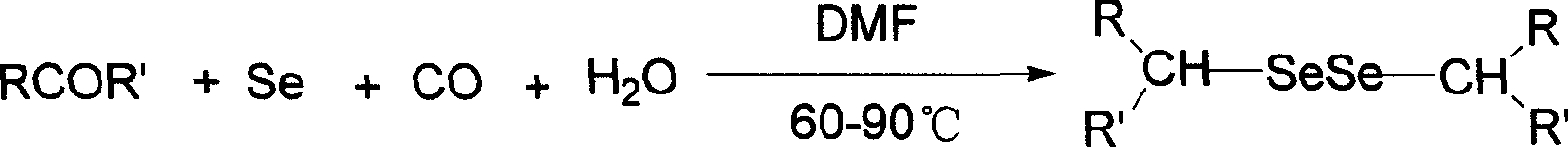Method of preparing alkyl diselenide
A technology of alkyl diselenide and cycloalkyl, applied in the field of alkyl diselenide, can solve the problems of complex operation, harsh conditions, long reaction time and the like, and achieve the effects of simple post-processing, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0015] The synthesis of embodiment 1 dicyclohexyl diselenide
[0016] Add cyclohexanone (2.5mmol), selenium (2.5mmol), water (2mL), and N,N-dimethylformamide 20ml into a 100ml three-necked flask, and feed it at a rate of 20-60mL / min under normal pressure CO, temperature controlled at 90°C, vigorous magnetic stirring for 7 hours. After the reaction is complete, stop passing CO, cool to room temperature, and stir in air for half an hour, add an appropriate amount of water, extract with ether three times, combine the extracts, and evaporate the solvent under reduced pressure. After passing through the column, the eluent was petroleum ether: ethyl acetate (10:1), and the eluent was concentrated to obtain the product with a yield of 74%, a yellow liquid.
Embodiment 2
[0018] The reaction temperature is room temperature (25° C.), the reaction time is 7 hours, the experimental method and steps are the same as in Example 1, and the yield is 0%.
Embodiment 3
[0020] The reaction temperature was 60° C., the experimental method and steps were the same as in Example 1, and the yield was 46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com