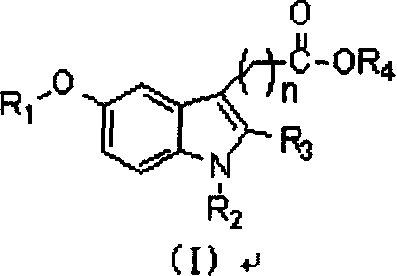
Indole kind compound with insuline sensitizing activity and its preparation method and use
A compound and indole technology, applied in the field of indole compounds and their preparation, can solve the problems of patient weight gain, liver toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1: 2-methyl-1-(4-chlorobenzoyl)-5-benzyloxy-1H-indole-3-acetic acid (compound 1)
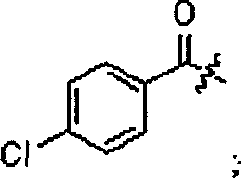
[0106] Dissolve 12.9g (40.8mmol) of sodium p-benzyloxyphenylhydrazine sulfonate in 576mL of water, raise the temperature to 55-65°C, add 476mL of ethanol after 15min, cool down to 20-25°C, add 5mL of p-chlorobenzoyl chloride, from the reaction solution Start timing when it thickens, stir for 1 hour, then raise the temperature to 75-80°C, and continue stirring for 1 hour. Cool down to 65±2°C, add 1N NaOH solution dropwise, adjust pH=9-10, stir for about 20 minutes until the pH remains unchanged, cool down to below 20°C, wash with suction to neutrality, and dry to obtain N-p-chlorobenzene Formyl-N-p-benzyloxyphenylhydrazine is a khaki solid.
[0107] 1 g (2.84 mmol) of N-p-chlorobenzoyl-N-p-benzyloxyphenylhydrazine was dissolved in 100 mL of absolute ethanol, and under ice bath conditions, dry HCl gas was introduced into the reaction liquid, and left at room temperature after satur...
Embodiment 2
[0109] Example 2: 2-methyl-1-(4-chlorobenzoyl)-5-benzyloxy-1H-indole-3-acetic acid methyl ester (compound 2)
[0110] 10mL CH 3 OH was cooled to -10°C in an ice-salt bath, and 0.6mL (15.9mol) SOCl was added dropwise 2 , Control the drop rate so that the internal temperature is less than -5°C. After dropping, add 2.52g (5.82mmol) of 2-methyl-1-(4-chlorobenzoyl)-5-benzyloxy-indole-3-acetic acid, raise the temperature to 25°C, and continue to stir until it is completely dissolved Then, stir at 0°C for another 1 h. The reaction solution was evaporated to dryness, dissolved in ethyl acetate, washed with water and brine, and dried over anhydrous sodium sulfate to obtain 0.19 g of an oily liquid. Petroleum ether:ethyl acetate=3:1 was used as the eluent, and the title compound was obtained as an oil 1.78g by silica gel column chromatography. Yield 68%. 1 H NMR (CDCl 3 ): δ=2.39(s, 3H), 3.47(s, 3H), 3.68(s, 2H), 5.07(s, 2H), 6.75(dd, J=8.8Hz, 2.4Hz, 1H), 6.86(d , J=8.8Hz, 1H), 7...
Embodiment 3
[0111] Example 3: 2-methyl-1-(4-fluorobenzoyl)-5-benzyloxy-1H-indole-3-acetic acid (compound 3)
[0112] Using 4-fluorobenzoyl chloride as a raw material, the title compound was prepared as a white solid according to the method of Example 1. The total yield is 17.8%. 1 H NMR (CDCl 3 ): δ=2.39(s, 3H), 3.68(s, 2H), 5.07(s, 2H), 6.75(dd, J=8.8Hz, 2.4Hz, 1H), 6.86(d, J=8.8Hz, 1H ), 7.05 (d, J = 2.4Hz, 1H), 7.26-7.47 (m, 7H), 7.74 (d, J = 8.6Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com