Preparation method of ammonia base formate kind agricultural pesticide hapten
A carbamate and nitrobenzene haloformate technology is applied in the field of new preparation of carbamate pesticide haptens, and can solve the problems of increasing the difficulty of operation, environmental pollution, increasing danger and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
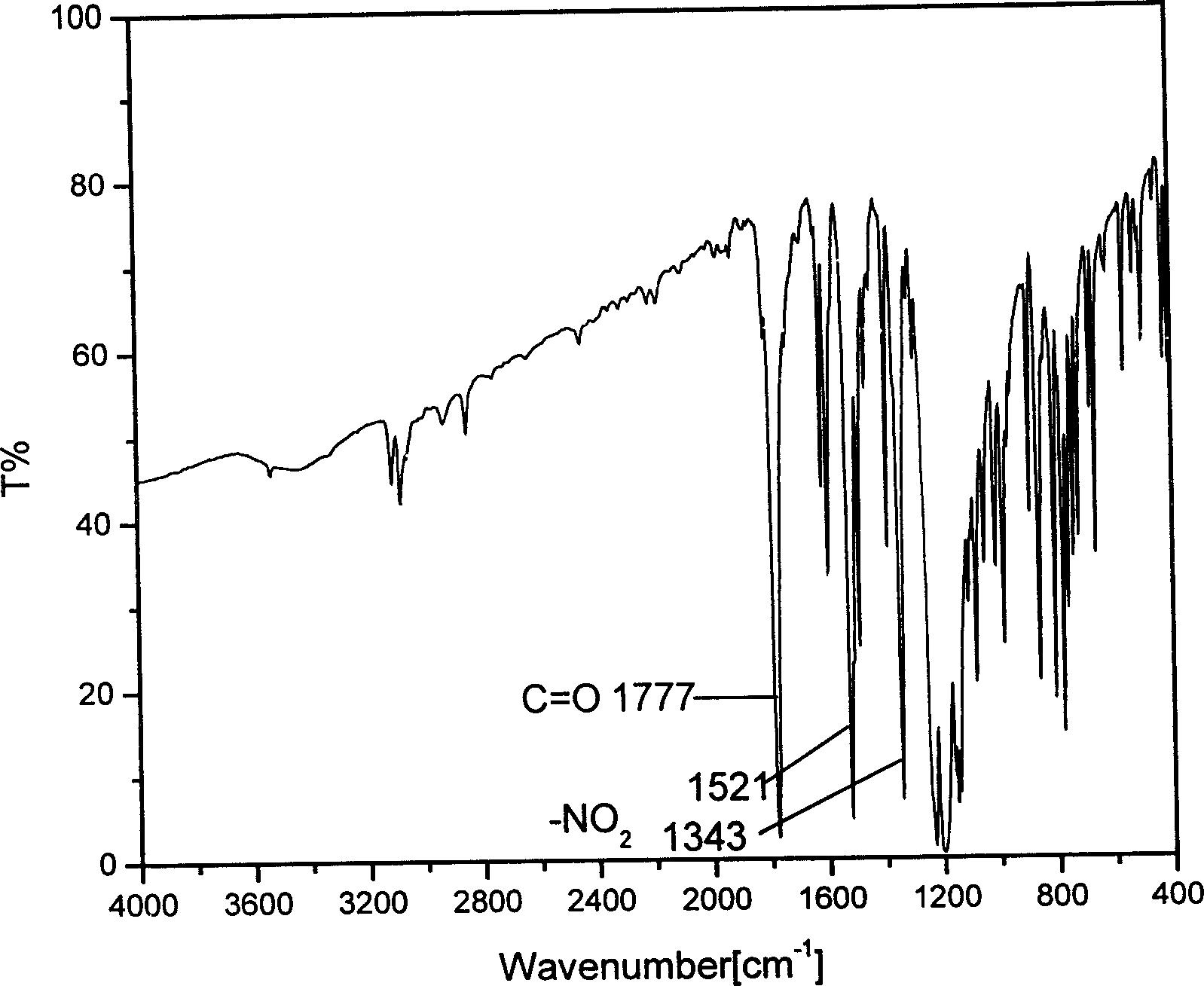
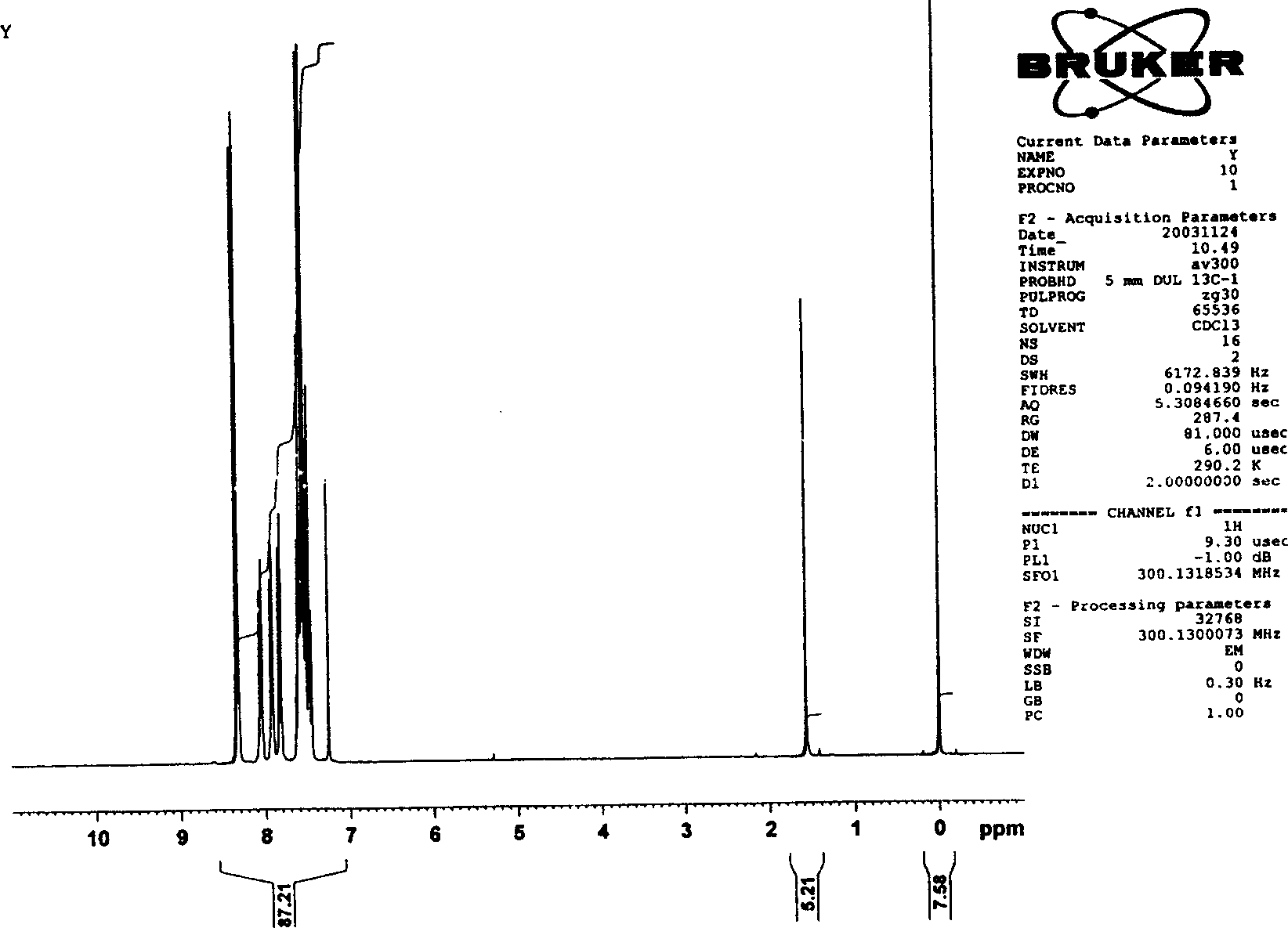
[0016] After dissolving 0.8g of naphthol in 20ml of anhydrous dichloromethane, add 0.58ml of anhydrous pyridine to form a mixed solution, and add dropwise anhydrous dichloromethane solution dissolved with 1g of p-nitrobenzene chloroformate in this mixed solution 15ml, the whole system was kept at -4°C-6°C. The reaction was protected with nitrogen and kept anhydrous. The reaction was followed by thin-plate chromatography (TLC) to determine the reaction time. Recrystallize from dichloromethane and ether. Light yellow 1-naphthyl p-nitrophenyl carbonate crystals were obtained with a yield of 80%. Characterized by infrared spectroscopy, there are characteristic peaks of carbonyl and nitro (attached figure 1 ). The correctness of the structure of the obtained product was further proved by proton nuclear magnetic resonance spectrum. (attached figure 2 )
[0017] Dissolve 1.2 mmol of 1-naphthyl p-nitrophenyl carbonate obtained in the previous step in tetrahydrofuran, dissolve ...
Embodiment 2
[0019] After 0.6g of 3-methylphenol was dissolved in 15ml of anhydrous dichloromethane, 0.58ml of anhydrous pyridine was added to form a mixed solution, and 1g of p-nitrophenyl chloroformate was added dropwise to the anhydrous dichloroformate in this mixed solution. Chloromethane solution 15ml, the whole system was kept at -4°C-6°C. The reaction was protected with nitrogen and kept anhydrous. The reaction was followed by TLC to determine the reaction time. Recrystallize from dichloromethane and ether. Light yellow 3-methylphenyl-p-nitrobenzene carbonate crystals were obtained with a yield of 87%. Characterized by infrared spectroscopy, there are characteristic peaks of carbonyl and nitro groups, and the correctness of the structure of the obtained product is further proved by hydrogen nuclear magnetic resonance.
[0020] Dissolve 1.2 mmol of 3-methylphenyl-p-nitrobenzene carbonate obtained in the previous step in tetrahydrofuran, dissolve 0.16 g of 6-aminocaproic acid in so...
Embodiment 3
[0022] After 0.8g of 3,4-xylenol was dissolved in anhydrous 15ml of methylene chloride, 0.58ml of anhydrous pyridine was added to form a mixed solution, and 1g of p-nitrophenyl chloroformate was added dropwise to the mixed solution. 15ml of anhydrous dichloromethane solution, and the whole system was kept at -4°C-6°C. The reaction was protected with nitrogen and kept anhydrous. The reaction was followed by TLC to determine the reaction time. Recrystallize from dichloromethane and ether. Light yellow 3,4-dimethylphenyl-p-nitrobenzene carbonate crystals were obtained with a yield of 85%. Characterized by infrared spectroscopy, there are characteristic peaks of carbonyl and nitro groups, and the correctness of the structure of the obtained product is further proved by hydrogen nuclear magnetic resonance.
[0023] Dissolve 1.2 mmol of 3,4-dimethylphenyl-p-nitrobenzene carbonate obtained in the previous step in tetrahydrofuran, dissolve 0.16 g of 6-aminocaproic acid in sodium bi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com