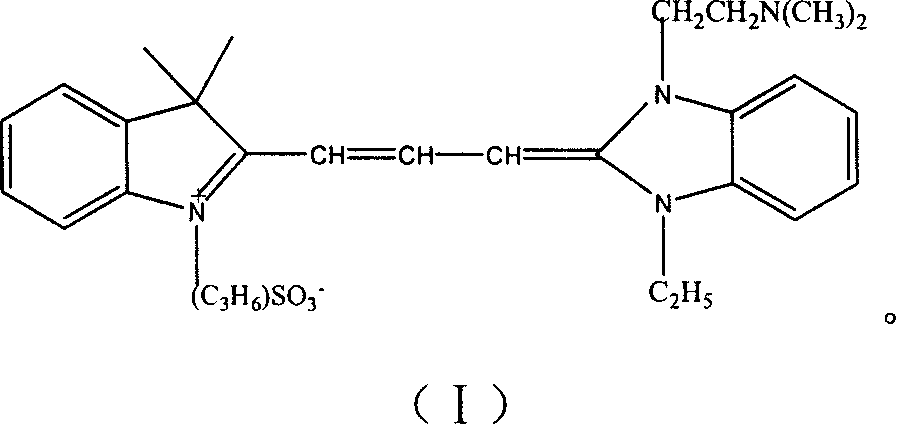
1,1-dimethyl-3-propyl sulfonate indole-1'-(n,n-dimethyl amine athyl amine)-3-ethyl imidazole carbocxyanine dyestuff, preparing process and use thereof
A dimethylaminoethylamino, propanesulfonic acid-based indolyl technology, applied in the field of photosensitive chemistry, can solve the problem of fog rise, difficult formation of silver chloride chemical non-stoichiometric lattice defects, inability to meet T-grain emulsions, etc. problem, to achieve the effect of increasing the sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 1,1-dimethyl-3-propanesulfonylindolyl-1'-(N,N-dimethylaminoethylamino)-3'-ethyl-imidazolyl-carbocyanine dye:
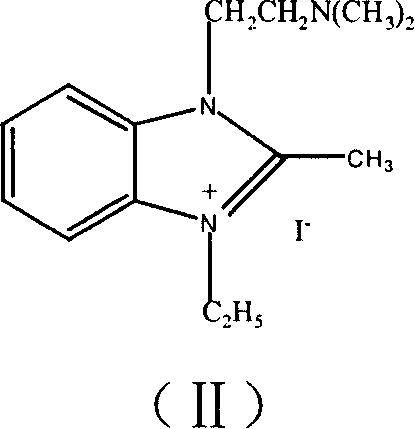
[0037] One. formula (II) compound, i.e. the preparation of 1-(N, N-dimethylaminoethylamino)-2-methyl-3-ethyl imidazole iodonium salt
[0038] 1) Preparation of o-(N,N-dimethylaminoethylamino)-nitrobenzene
[0039] In a 500ml three-necked flask, add 57.2g (0.283mol) of 2-chloronitrobenzene, 33.2g (0.283mol) of N,N-dimethylethylenediamine and 70.0g of anhydrous sodium acetate, and the reactants are mixed After that, it turns red soon, and is heated to 140°C to 150°C under stirring, and kept for 10 hours. As the reaction proceeded, the reaction mixture gradually became viscous. After the reaction was completed, the reaction mixture was naturally cooled to room temperature, 500ml of water was added thereto for dilution, and then 30ml of hydrochloric acid (30wt%) was added, at this moment, unreacted raw material 2-chloronitrobenzene was precipitated...
Embodiment 2
[0069] Spectral sensitization of compounds of the present invention in cubic silver chlorobromide microcrystalline emulsion:
[0070] 1. Preparation of cubic silver chlorobromide microcrystalline emulsion:
[0071] In a container with a volume of 2 liters, add 500 ml of deionized water, 8 g of gelatin, 0.15 g of sodium chloride, and 1.2 g of potassium iodide in advance, and heat to a constant temperature of 65° C. Add 12.5 milliliters of 0.1 mol / liter concentration of silver nitrate aqueous solution and 12.5 milliliters of 0.1 mol / liter concentration of sodium chloride solution under high-speed stirring, then add 100 milliliters of 4 mol / liter concentration of silver nitrate solution and 100 milliliters of A mixed solution of sodium chloride and potassium bromide at a concentration of 4 mol / liter (wherein the molar ratio of sodium chloride to potassium bromide=5:1). After the reaction, cool down to 30°C, then add 20 milliliters of 10wt% sodium polystyrene sulfonate solution a...
Embodiment 3
[0079] Spectral sensitization of compounds of the present invention in cubic silver bromoiodide microcrystalline emulsion:
[0080] 1. Preparation of cubic silver bromide iodide microcrystalline emulsion:
[0081] In a container with a volume of 4 liters, add 1000 ml of deionized water, 20 g of gelatin, 1.0 g of sodium chloride, and 2.5 g of potassium iodide in advance, heat to 70° C., and keep the temperature constant. Under high-speed stirring, add 10.0 milliliters of 0.4 mol / liter concentration of silver nitrate aqueous solution and 10.0 milliliters of 0.4 mol / liter concentration of potassium iodide and potassium bromide solution in 1 minute (wherein the molar ratio of potassium iodide and potassium bromide=1:5) Then add the silver nitrate solution of 92 milliliters of 2.0 mol / liter concentration and the mixed solution of potassium iodide and potassium bromide of 92 milliliters of 2.0 mol / liter concentration (wherein the mol ratio=1:5 of potassium iodide and potassium bromi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com