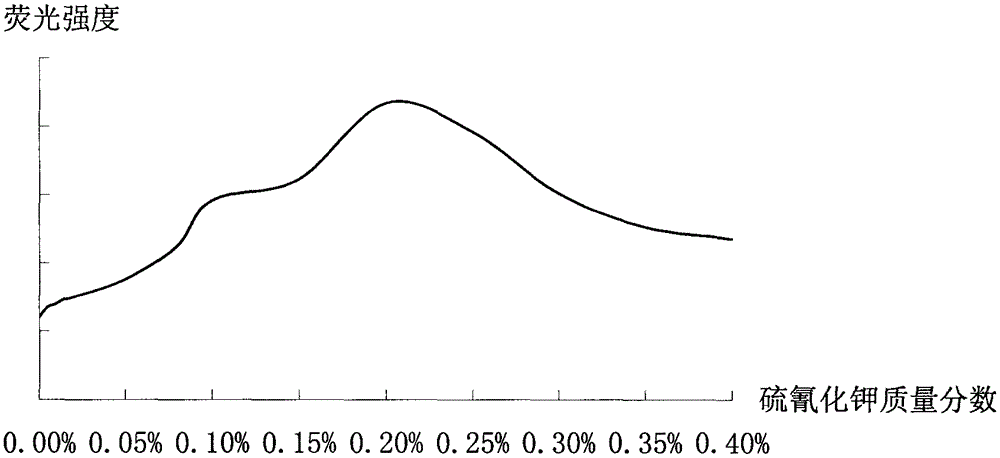
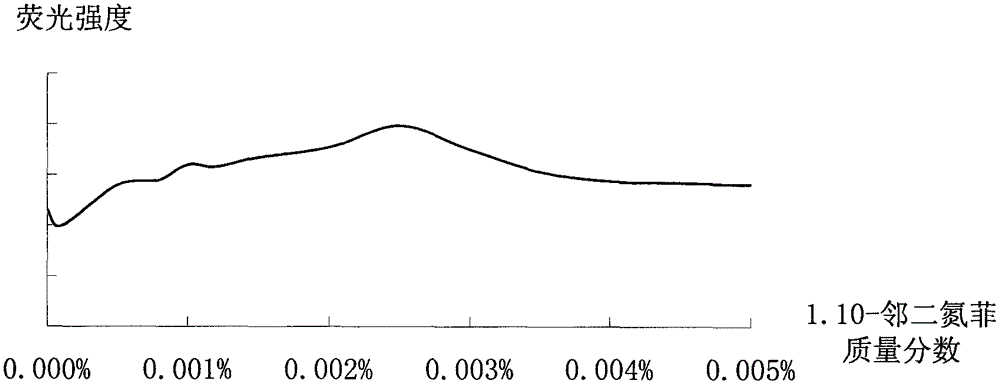
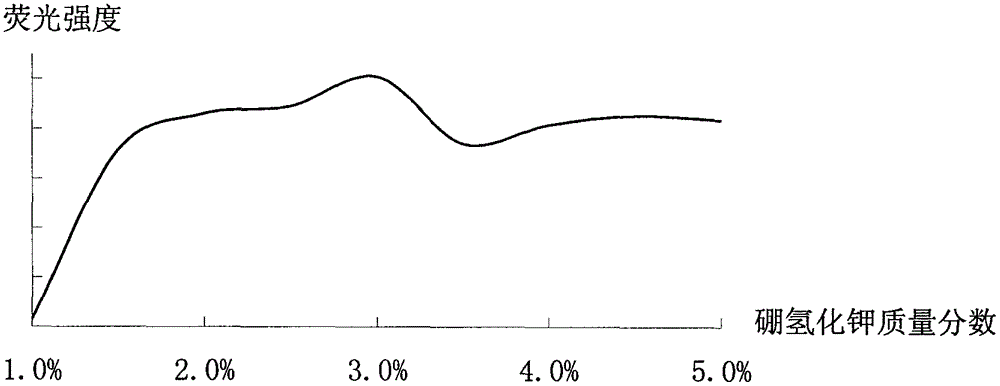
Improved method for detecting zinc through atomic fluorescence spectrophotometer
An atomic fluorescence spectrophotometry, reducing agent technology, applied in the field of analysis and detection, can solve the problems of lack of stability of experimental data, poor reproducibility of experimental results, existence of memory effect, etc. little interference effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Weigh 10 grams of crushed fresh cabbage sample into a 50mL porcelain crucible, add 1mL of 10% phosphoric acid, transfer it to a muffle furnace after carbonization on a low fire, ash at 500°C for 8 hours, take it out and cool it, add a small amount of 10% hydrochloric acid to dissolve And transfer it to a 50mL volumetric flask, add 1.25mL hydrochloric acid and 10mL 2.0% potassium thiocyanate solution, dilute to the mark with water and shake well, and do reagent blank and standard addition recovery experiments. Pipette 1mL, 2mL, 5mL, 8mL, 10mL of 1000μg / L zinc standard solution into a 50mL volumetric flask, add 1.25mL of hydrochloric acid and 10mL of 2.0% potassium thiocyanate solution, dilute to the mark with water and shake well, Prepare working curves of zinc standard solutions with mass fractions of 20 μg / L, 50 μg / L, 80 μg / L, 100 μg / L, and 200 μg / L, respectively. The fluorescence intensity of the working curve of the standard solution and the sample solution was measu...
Embodiment 2
[0040]Weigh 0.3 g of air-dried, crushed and sieved soil into a 50 mL polytetrafluoroethylene cup, add a small amount of water and 10 mL of hydrochloric acid to moisten the soil, volatilize at low temperature until about 2 mL remains, then cool, add 5 mL of nitric acid, 5 mL of hydrofluoric acid and 3 mL of perchloric acid Heat the flying silicon, add nitric acid hydrofluoric acid perchloric acid in proportion to continue flying silicon according to the digestion situation, until the content of the crucible is viscous, remove and cool, add 1mL nitric acid to dissolve the residue, transfer to a 50mL volumetric flask, add 1.25 Make mL of hydrochloric acid and 10 mL of 2.0% potassium thiocyanate solution, dilute to the mark with water and shake well, and do reagent blank and standard addition recovery experiments. Pipette 1mL, 2mL, 5mL, 8mL, 10mL of 1000μg / L zinc standard solution into a 50mL volumetric flask, add 1.25mL of hydrochloric acid and 10mL of 2.0% potassium thiocyanate s...
Embodiment 3
[0042] Take 25mL of tap water in a 50mL volumetric flask, add 1.25mL of hydrochloric acid and 10mL of 2.0% potassium thiocyanate solution, make the volume up to the mark with water and shake well, and do reagent blank and standard addition recovery experiments. Pipette 1mL, 2mL, 5mL, 8mL, 10mL of 1000μg / L zinc standard solution into a 50mL volumetric flask, add 1.25mL of hydrochloric acid and 10mL of 2.0% potassium thiocyanate solution, dilute to the mark with water and shake well, Prepare working curves of zinc standard solutions with mass fractions of 20 μg / L, 50 μg / L, 80 μg / L, 100 μg / L, and 200 μg / L, respectively. The fluorescence intensity of the working curve of the standard solution and the sample solution was measured with an atomic fluorescence spectrometer. After calculation, the zinc content in tap water was 54.7 μg / L, and the recovery rate was 97.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com