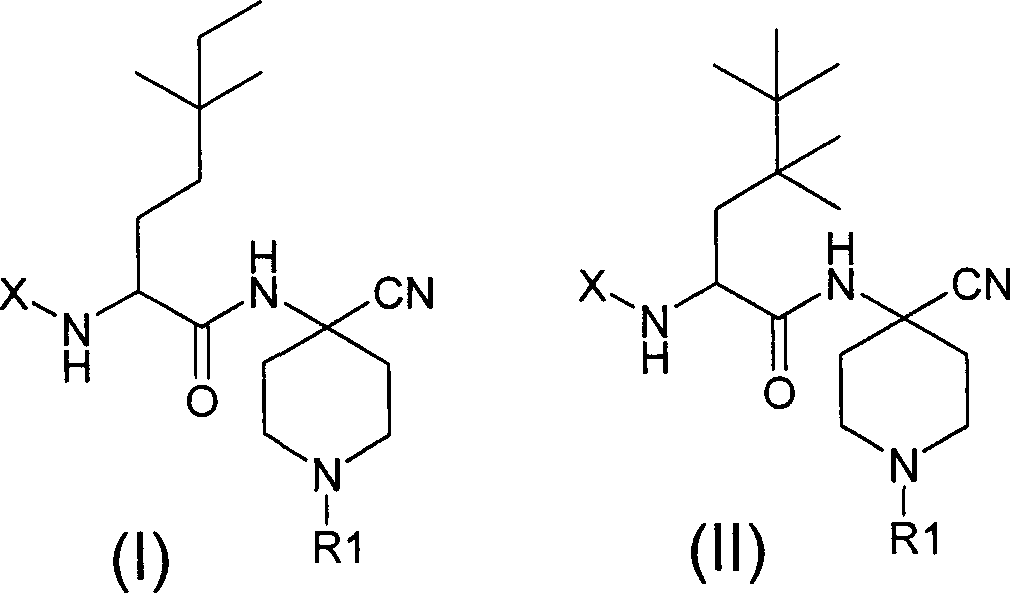
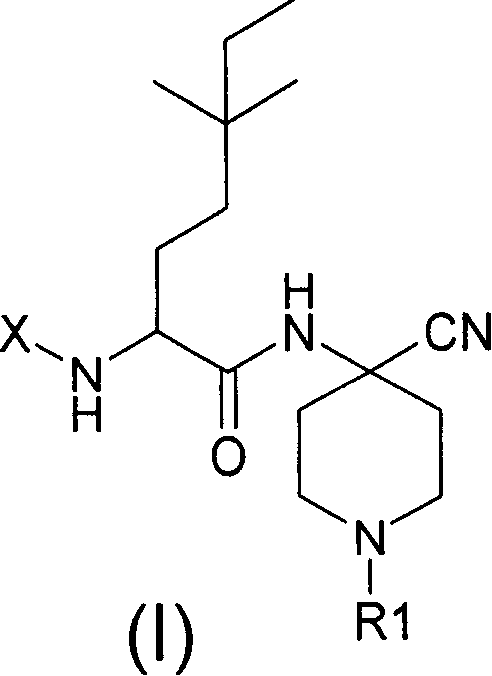
Cathepsin S inhibitors
A compound, selected technology, applied in the direction of anti-inflammatory agents, drug combinations, non-central analgesics, etc., can solve problems such as increased toxicity, non-target enzyme inactivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1: Synthesis of 2-tert-butoxycarbonylamino-4,4,5,5-trimethyl-hexanoic acid
[0095]
[0096]
[0097]
[0098] Lithium diisopropylamide (LDA) (1.5 M solution in cyclohexane / THF / ethylbenzene) (106 mL, 160 mmol, 1.15 equiv) was injected into a 1000 mL round bottom flask under an argon atmosphere. Dry THF (150 mL) was added and the mixture was cooled to -78°C with a dry ice / acetone bath. 3,3-Dimethyl-butyric acid ethyl ester (20 g, 23.3 mL, 139 mmol, 1.0 equiv) was added dropwise via syringe over 10 minutes, followed by stirring at -78°C for 1 hour. Iodomethane (9.5 mL, 152 mmol, 1.1 equiv) was added dropwise by syringe over 10 minutes, and the creamy mixture was stirred at -78°C for 1 hour to give a very thick mixture. The dry ice bath was removed and replaced with a 0 °C ice bath. An additional 150 mL of dry THF was added, followed by additional LDA (106 mL, 160 mmol, 1.15 equiv). The resulting mixture was stirred for 10 minutes, then the round bottom...
Embodiment 2
[0104] Example 2: Synthesis of 2-tert-butoxycarbonylamino-5,5-dimethyl-heptanoic acid
[0105]
[0106]
[0107] 3,3-Dimethyl-pent-4-enoic acid methyl ester (20.0 mL, 126 mmol, 1.00 equiv) was carefully added via pipette to the 4 (3.63 g, 96 mmol, 0.76 equiv) in 500 mL of anhydrous diethyl ether in a 2 L flask (cooled by an ice-water bath). The reaction mixture was allowed to warm to room temperature overnight with stirring, then quenched by the slow addition of saturated potassium sodium tartrate solution (150 mL). The mixture was diluted with ether (200 mL), and the organic layer was separated and dried (MgSO 4 ), concentrated to give 3,3-dimethyl-pent-4-en-1-ol (11.0 g, 76% yield) as a colorless liquid. This material was used without further purification; 1 H NMR (CDCl 3 , 400MHz) δ1.00(s, 6H), 1.58(t, J=7.3Hz, 2H), 2.07(s, 1H), 3.59(t, J=7.3Hz, 2H), 4.89-4.94(m, 2H ), 5.80 (dd, J=17.3, 14.1, 1H).
[0108] Anhydrous DMSO (17.1 mL, 241 mmol, 2.5 eq) was added dro...
Embodiment 3
[0112] Example 3: Synthesis of (S)-2-tert-butoxycarbonylamino-5,5-dimethyl-heptanoic acid
[0113]
[0114] R,R-DIPAMP cyclooctadiene Rh(I) tetrafluoroborate (190 mg, 0.25 mmol, 0.04 equiv) was added to (Z)-2-benzyloxycarbonylamino-5 in a Paar hydrogenation flask, A solution of 5-dimethyl-hept-2,6-dienoic acid methyl ester (2.00 g, 6.30 mmol, 1.00 equiv) in dry methanol (20 mL). The reactor was evacuated and flushed 3 times with hydrogen, then shaken vigorously under 50 psi hydrogen overnight. The reaction mixture was concentrated in vacuo, then filtered through a plug of silica gel using ethyl acetate in hexanes as the gradient eluent to afford (S)-2-benzyloxycarbonylamino-5,5- Dimethyl-heptanoic acid methyl ester (1.63 g, 81% yield); 1 H NMR (CDCl 3 , 400MHz) δ0.74-0.90(m, 9H), 1.10-1.30(m, 4H), 1.58-1.70(m, 1H), 1.75-1.90(m, 1H), 3.75(s, 3H), 4.30- 4.40(m, 1H), 5.14(s, 2H), 5.24-5.33(m, 1H), 7.30-7.37(m, 5H); [α] 20 D =+15.57c=2.00, CHCl 3 .
[0115] In a Paar re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chirality | aaaaa | aaaaa |
| chirality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com