Vinorelbine preparation method
A technique for vinorelbine and dehydrated vinblastine, which is applied in the field of synthesis and purification of vinorelbine, can solve problems such as high market price, and achieve the effects of less solvent consumption, improved reaction rate, and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
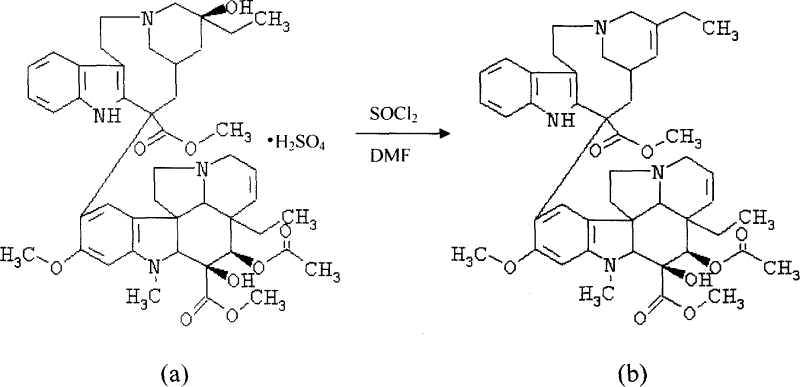
[0024] Embodiment 1: Vinblastine sulfate (a) prepares dehydrated vinblastine (b)
[0025]
[0026] Weigh 2 g of vinblastine sulfate, dissolve it in 30 ml of anhydrous N, N-dimethylformamide under the protection of nitrogen, and drop into N, N-dimethylformamide solution of thionyl chloride (4 ml of chlorinated Dissolve sulfoxide in 10ml of N,N-dimethylformamide), ultrasonic cavitation reaction at room temperature for about 80min, stop the reaction when TLC tracked to no raw material point, add 160ml of ice water, adjust pH to 9 with ammonia water, extract with ether 3 times , 120ml each time, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure at 40°C, N 2 Methanol recrystallization under protection gave 1.26 g of dehydrated vinblastine with a purity of 82% and a yield of 59.2%.
Embodiment 2
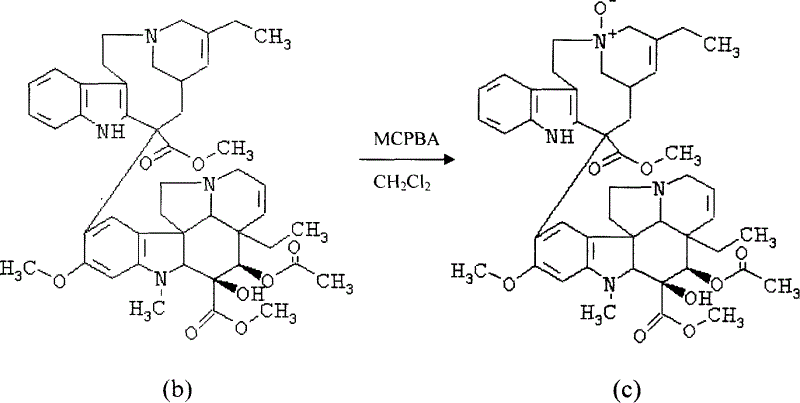
[0027] Embodiment 2: Dehydrovinblastine (b) prepares dehydrovinblastine-N-oxide (c)
[0028]
[0029] Weigh 1.25 g of dehydrated vinblastine, dissolve it in 37 ml of anhydrous dichloromethane, add a methylene chloride solution of m-chloroperoxybenzoic acid (0.37 g of m-chloroperoxybenzoic acid is dissolved in 18.5 ml of methylene chloride), and Ultrasonic cavitation reaction for 10min, pour the reaction solution into 11ml of saturated sodium carbonate solution, extract with 185ml of chloroform, wash with an equal volume of saturated sodium chloride solution, dry the chloroform layer with anhydrous sodium sulfate, filter, and recover under reduced pressure at 40°C Solvent, the residue was obtained.
Embodiment 3
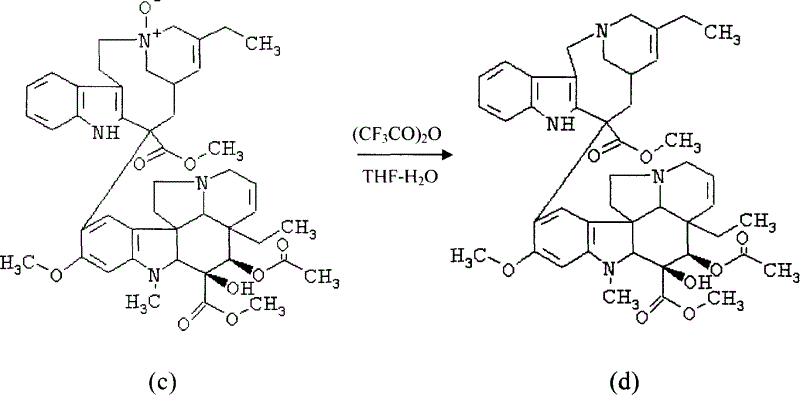
[0030] Example 3: Preparation of Vinorelbine from Dehydrated Vinblastine-N-Oxide
[0031]
[0032] Dissolve the above residue in 7.4ml of anhydrous dichloromethane under the protection of nitrogen, add 1ml of trifluoroacetic anhydride at 0°C, keep warm for 30min by ultrasonic cavitation reaction, and recover the solvent under reduced pressure (without heating) to obtain the residue. Dissolve in 29.6ml of tetrahydrofuran, add 0.11ml of water, conduct ultrasonic cavitation reaction at room temperature for 30min, pour the reaction solution into 25ml of saturated sodium chloride solution, extract with 150ml of chloroform, wash with saturated sodium chloride solution until pH = 7, and wash the chloroform layer with Dry over anhydrous sodium sulfate, filter, and recover the solvent from the filtrate under reduced pressure to obtain a residue of 0.73 g with a purity of 71% and a yield of 42.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com