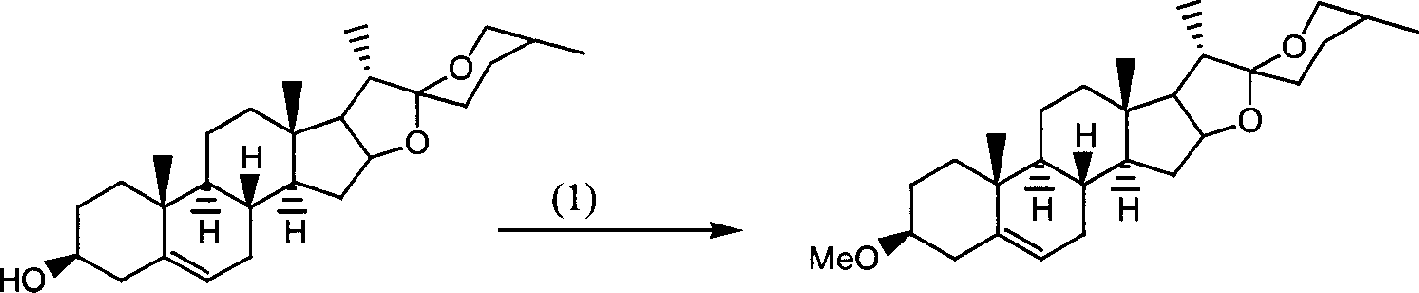Synthesis process of chlesterol and its intermediate
A synthetic method and cholesterol technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of unsuitability for industrial production and low yield, and achieve the effect of high yield, high yield and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment one: the synthetic method of cholesterol, comprises the following steps:
[0040] [Protection of Hydroxyl]: 6.0 grams (0.15mol) of 60% sodium hydride is placed in a 1L three-necked flask, 500ml of anhydrous treated tetrahydrofuran is added, and 41.6 grams (0.1mol) of diosgenin is added in batches under stirring Wherein, after the addition, continue to stir and react for 15 minutes, then add 18.9 grams (0.15mol) of dimethyl sulfate to the mixed solution, stir the reactant at room temperature overnight, add methanol to the reaction system to decompose excess sodium hydride, until the addition No gas was generated from methanol, the mixed solution was poured into 2L of water, cooled and filtered, and the crude product was recrystallized with methanol to obtain 40.6 g of white needle-like crystals. Melting point: 184.5-185.0°C, yield: 95.3%.
[0041] [Ring-opening reaction]: 20 grams (0.046mol) of diosgenin 3-methyl ether and 300 grams (4.6mol) of high-quality z...
Embodiment 2
[0047] Embodiment 2: A kind of synthetic method of cholesterol, take diosgenin as raw material, carry out following treatment:
[0048] (1) Protection of hydroxyl group: in the presence of an organic solvent, treat diosgenin with sodium hydride or potassium hydride to obtain sodium diosgenate or potassium diosgenate, and the reaction molar ratio is 1:1.2-2.0; Then react with dimethyl sulfate, the reaction molar ratio is 1:1.0~2.0, remove excess sodium hydride or potassium hydride with methanol, cool and filter in water, and recrystallize to obtain diosgenin-3-methyl ether;
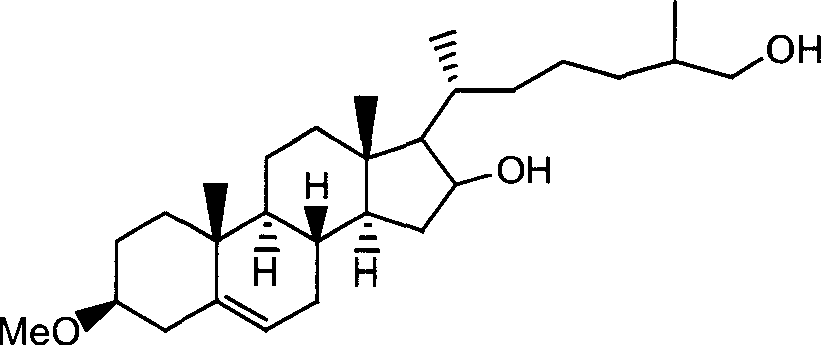
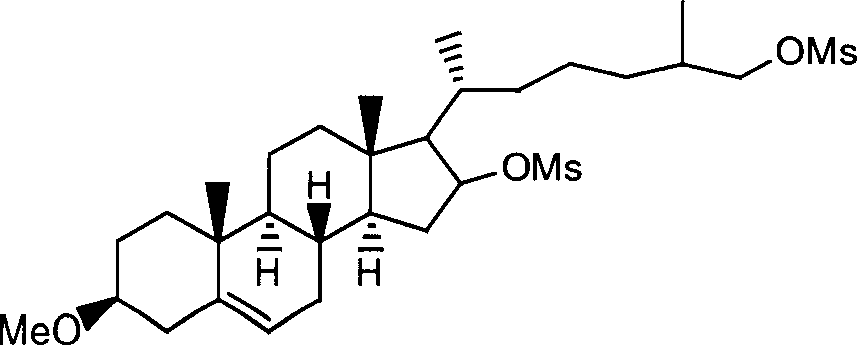
[0049] (2) Ring-opening reaction: the obtained diosgenin-3-methyl ether is added together with zinc powder in absolute ethanol, the suspension is heated to reflux, concentrated hydrochloric acid is slowly added dropwise, and the reaction product is suction filtered and recrystallized, To obtain 16,26-dihydroxycholesterol-3-methyl ether, the molar ratio of diosgenin-3-methyl ether, hydrochloric acid and zin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com